Translate this page into:
Immunological safety assessment of a single and repeated intra-articular administration of xenogeneic equine umbilical cord mesenchymal stem cells under field conditions in young healthy dogs: A randomized double-blind placebo-controlled study

*Corresponding author: Elena Garcia-Pedraza, Department of Animal Medicine and Surgery, Veterinary Teaching Hospital, Veterinary Faculty, Complutense University of Madrid, Madrid, Spain. elgapedraza@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garcia-Pedraza E, de Miguel AG, Gomez de Segura IA, Portolés AP. Immunological safety assessment of a single and repeated intra-articular administration of xenogeneic equine umbilical cord mesenchymal stem cells under field conditions in young healthy dogs: A randomized double-blind placebo-controlled study. Res Vet Sci Med 2022;2:2.
Abstract
Objectives:
The objective was to study the cellular immune response of equine umbilical cord mesenchymal stem cells (EUC-MSCs) in healthy dogs after a single and repeated intra-articular administration versus placebo in the right knee.
Material and Methods:
Sixteen dogs were randomized into two groups of eight dogs that received two intraarticular administrations of placebo or EUC-MSCs on day 0 and on day 28. Blood samples for the analysis of cellular response were obtained from the cephalic vein on days 0 for baseline data, 14, 28 (before the second administration), 42, and 56. A cellular response assay was made through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT tetrazolium) method.
Results:
No cytotoxic reaction occurred between lymphocytes and EUC-MSC demonstrating the safety of EUCMSCs administration in dogs.
Conclusion:
Overall these results suggest that the administration of EUC-MSCs can be considered as safe.
Keywords
Dog
MTT Tetrazolium assay
Stem cells
Xenogeneic and cellular response
INTRODUCTION
Adult stem cells, such as mesenchymal stem cells (MSCs), are considered a treatment option for degenerative or immune-mediated diseases. They can differentiate into different cell types in vitro such as chondrocytes; they have a high in vitro expansion potential[1] and can be easily isolated from adult tissues. Equine umbilical cord MSCs (EUC-MSCs) are obtained from the umbilical cord matrix or Wharton’s Jelly through enzyme digestion or explant technique. These cells are suggested to be more therapeutically active than other MSCs. MSCs, adult stem cells, are fibroblast-like, adherent cells that can differentiate in vitro into functional cell types including chondrocytes, osteocytes, adipocytes, and myocytes.[2,3] Surface markers characterization shows expression of CD105, CD73, and CD90.[4] MSCs are used in tissue engineering due to their high in vitro expansion potential, self-renewal capacity, and multipotentiality.[1] They can be isolated from adult tissues, such as umbilical cord, after full-term delivery, from a sample that would be discarded. The collection is safe, easy, ethical, noninvasive, and painless.[1,5] EUC-MSCs are obtained from a mucoid connective tissue, Wharton’s Jelly, that surrounds the two arteries and the vein of the umbilical cord.[4] It is suggested that UC-MSCs are much more proliferative, immunosuppressive, and therapeutically active than those isolated from bone marrow or adipose tissue.[6]
EUC-MSCs are immune-privileged. They have MHC-Class I expression and MHC-Class II low expression levels. They do not express T-cell costimulatory molecules at basal level.[1,7,8] They secrete interleukin 10 and nitric oxide, which inhibit T-cell proliferation.[5] It is suggested that prostaglandin E2 is the principal mediator of its immunomodulatory properties.[3,9] These data indicate that EUC-MSCs show a low immunogenic profile, which implies its recognition and tolerance by the immune system. Some studies suggest that EUC-MSCs can be administered twice without eliciting a measurable cellular immune response.[7]
In veterinary regenerative medicine, clinical studies of allogeneic and xenogeneic MSCs transplantation aiming the therapy of tendon injury, bone fracture, spinal cord injury,[10,11] superficial digital flexor tendinopathy,[12] superficial digital flexor tendon,[2] brain ischemia, cardiac infarction, and osteoarthritis[13,14] showed improved results with MSC treatment compared to conventional therapies. In osteoarthritis, the current treatment involves the use of nonsteroidal anti-inflammatory drugs that alleviate discomfort, decelerate tissue damage, and/or improve joint function. MSCs treatment restores physiological balance and enhances healing by promoting migration of endogenous repairing cells, differentiating toward mesodermal lineages, and suppressing immune reactions.[3,10,15]
Recent studies show that MSCs xenogeneic transplantation in canine in vivo models is a safe and immunocompatible procedure.[16] The repeated xenogeneic cell transplantation with human adipose-derived (AD) MSCs in the nonimmunosuppressed in vivo dog model of Duchenne muscular dystrophy was well tolerated with no negative side effects or tumors. Furthermore, the intravenous administration of human AD-MSCs to dogs for the treatment of experimentally induced atrial injury did not changed the composition of peripheral blood lymphocytes of the recipient dogs, indicating immunocompatibility.[5] Furthermore, swine ADMSCs injection into canine stifle joints with osteoarthritis did not cause inflammatory or allergic reactions and with an efficacy comparable to autologous treatments.[10]
In horses, the repeated administration of EUC-MSC does not produce a cellular immune response potentially leading to cell apoptosis and death.[8,12] To test the clinical relevance and xenogeneic safety, the objective of this randomized, double-blinded clinical trial was to assess the cellular response, after a single and repeated intra-articular EUC-MSC administration, compared to placebo, to young healthy dogs under field conditions.
MATERIAL AND METHODS
EUC-MSCs isolation, culture, and formulation of dosage
EUC-MSCs were manufactured under good manufacturing practices. They were isolated from EUC of healthy donors. This information about the manipulation of the tissue is confidential. The tissue was mechanically dissected and enzymatically digested. The obtained cell suspension was seeded and expanded until passage 4. EUC-MSCs were cultivated in medium Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (FBS), L-Glutamine (200 mM), D-Glucose (4.5 g/L), and antibiotics streptomycin/penicillin. At passage 4, the cell suspension was dispensed in a sterile 2 mL vial, containing 7.5 × 106 cells/mL ± 20%.
To ensure a high quality and safety of the cellular products throughout the manufacturing period, cellular number, viability, morphology, purity, identity, and accumulative doubling population were established as quality controls, as well as sterility, endotoxins, and mycoplasma tests. The manufacturing process is confidential, so details cannot be provided at this time.
Target animal safety design
The study was conducted following regulatory requirements and the Veterinary Investigational Product of the study was authorized by the Spanish Medicines Regulatory Authority (Agencia Española de Medicamentos y Productos sanitarios). Typical toxicity guidelines (GL43)[17] for chemicals or typical immunological guidelines (GL44)[18] have not been applied to the design of this study because they cannot be completely applicable to stem cell products due to its known high therapeutic index. The Experts Group on Veterinary Novel Therapies (ADVENT) published a document as a guide for applicants to design and conduct appropriate tests applicable to these new therapies.[19]
The present study was a masked (blinded), parallel group, randomized, and negative control trial and was carried out following the Good Clinical Practice guidelines, VICH 9 Guidelines.[20] The study was closely monitored to assure the animal welfare, the quality of the data, and the protection of the investigators involved in the study. Before inclusion, an informed consent was signed by the responsible person, where all the dogs, police dogs, were housed. A screening visit was performed before inclusion that included a clinical examination, an orthopedic assessment, blood tests, and confirmation of a validated pain scale score of 0 (no Pain) for the short-form of the Glasgow Composite Measure Pain Scale[21] as baseline determination. A total of 16 dogs were enrolled in the present study complying with all the inclusion criteria (healthy dogs with normal hematology and biochemistry tests, 1–4 years old on inclusion, of either sex, of any breed, weighing between 20 and 40 kg) and exclusion criteria (not healthy dogs, dogs with lameness, biochemical or hematological signs of illness, total protein levels ≥8.5 mg/dl as a possible indicator of infection with Leishmania spp. or autoimmune disease, pregnant or lactating females). The dogs were randomly allocated into two groups of eight animals for EUC-MSCs or placebo (Saline). Neither the investigator administrating the treatments and assessing the dogs during the study nor the person responsible for the dogs was aware of the treatment administered to them. Therefore, the study was double-blinded. In the treatment group, eight dogs received a single and repeated dose of 7.5 × 106 cells ± 20% of EUC-MSCs in 1 mL of excipient. In the placebo group, eight dogs received a single and repeated dose of saline solution.
The first dose was administered on day 0 in the right knee, by a lateral approach after aseptic preparation of the injection site. After the product administration, the dogs were clinically assessed on a daily basis for 7 days and then weekly on days 14, 21, and 28, when a second intra-articular injection of EUC-MSCs or placebo, as appropriate, was administered in the right knee. All the visits included the same assessments that were performed in the screening visit and the scheduled blood tests along the study.
This intensive care period consisted in a daily evaluation of the dog during the 7 days following the injections. A complete clinical and orthopedic evaluation was performed to detect any possible adverse event after the administration of the product. After the second administration, another daily follow-up took place from day 29 + 1 until day 34 + 1, so the weekly assessment on day 35 was coincident in time with the last day of the week of follow-up after the second administration. After that, weekly evaluations were performed on days 42, 49, 56, and 63. Blood samples for analysis of cellular response were obtained from the cephalic vein on days 0 (baseline), 14, 28 (before the second administration), 42, and 56.
The immunological response was investigated after the single and repeated administration through the mixed leukocyte reaction (MLR) as an evaluation of the cellular response [Table 1 for study scheduled assessments].
| Activity | Screening visit | Day 0 | Daily follow-up (day 1–6) | Day 7 | Day 14 | Day 21 | Day 28+1 | Daily follow-up (day 29–34) | Day 35 | Day 42 | Day 49 | Day 56 | Day 63 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochemistry and hematology | X | X (Day 2) | X | X (Day 30) | X | ||||||||
| Intra-articular treatment | X | X | |||||||||||
| Clinical examination | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Orthopedic exploration of the treated joint | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Glasgow Scale assessment | X | X | X | X | X | X | X | X | X | X | X | X | |
| Humoral response | X | X | X | X | |||||||||
| Cellular response | X | X | X | X | X | ||||||||
| Registration adverse events | X | X | X | X | X | X | X | X | X | X | X | ||
| Registration concomitant drugs | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Study completion | X |
MLR as an evaluation of the cellular response
The lymphocyte fraction (peripheral blood mononuclear cells or PBMCs) was obtained through separation by density gradients of blood extracted from the treatment/placebo dogs on scheduled days. PBMCs were cocultured with EUCMSCs. Three days later, dimethyl sulfoxide (DMSO) was added in the wells that corresponded to the death control. Afterward, the MTT tetrazolium assay was performed, and the absorbance was recorded with a plate reader.
Isolation and culture of the lymphocyte fraction (PBMCs)
Blood was extracted from EUC-MSCs treated dogs in heparin containing tubes at days 0 (screening visit), 14, 28 (before second administration), 42, and 56. Twenty-four hours after every blood extraction, isolation of the lymphocyte fraction (PBMCs) was performed through separation by density gradients with Ficoll, in which the collected blood was diluted in phosphate-buffered saline (PBS) ×1 and slowly added in Pancoll human (Lymphocyte Separation Medium, density 1.077 g/mL) being careful not to mix them. Then, it was centrifuged at 800× g for 30 min and the lymphocyte fraction (PBMCs) was removed. PBMCs were washed twice in PBS ×1 and cultured in Roswell Park Memorial Institute Medium (RPMI) 1640 supplemented with 20% FBS, L-Glutamine (200 mM), and antibiotics streptomycin/penicillin. Afterward, they were incubated at 37°C with 5% CO2.
Coculture of PBMCs with their respective MSCs
Twenty-four hours after the lymphocyte fraction isolation, PBMCs were collected and centrifuged at 350 xg for 10 min. Then, a PBMC suspension was prepared with 106 cells in 1 mL of complete RPMI 1640. Later, PBMC suspensions from each dog were cocultured in 12-well plates, previously seeded with EUC-MSCs, at a ratio 10:1 (PBMC: EUC-MSC).[8] At the same time, PBMC suspension from the placebo group was cocultured with EUC-MSCs, as no reaction was expected. Afterward, they were left overnight (o/n) at 37°C, 5% CO2.
In parallel to the study samples, control wells were included in each plate:
Positive control of EUC-MSC activity. Wells were seeded only with EUC-MSC in culture media.
Negative/death control of EUC-MSC activity. Wells were seeded with EUC-MSC and DMSO. The DMSO concentration was determined in a previous experimental set-up phase of the MLR assay by testing 0.1, 0.5, 1, 2.5, 5, 7.5, and 10% of DMSO and obtaining the percentage of activity that has decreased. To assess a suitable DMSO concentration for EUC-MSCs death, different concentrations of DMSO, 0.1, 0.5, 1, 2.5, 5, 7.5, and 10% were added to the wells previously seeded with EUC-MSCs. After 24 hours of incubation at 37°C and 5% CO2, an MTT assay was performed [Figure 1].

- DMSO toxicity curve expressed as mean. (Basal n=4, 0.1% n=3, 0.5% n=3; 1% n=4, 2.5% n=4, 5% n=4, 7.5% n=1 and 10% n=1). DMSO: Dimethyl sulfoxide.
Seventy-two hours later, 5% DMSO was added to the wells that corresponded to the death control.
MTT Tetrazolium assay
The MTT tetrazolium assay is a rapid, quantitative, colorimetric method for assessing cellular proliferation, metabolic activity, and viability. The biomolecular basis is that the mitochondrial enzyme succinate dehydrogenase reduces MTT into formazan that accumulates as an insoluble precipitate within the cells. Formazan needs to be solubilized with DMSO before recording the absorbance with a plate reader. This signal is proportional to the number of viable cells present and, when cells die, they quickly lose the ability to convert the substrate into product.
In the laminar flow cabinet without light, 1 mL of medium was removed and the MTT solution was added to a final concentration of 0.5 mg/ml. The 12-well plates were placed in the incubator at 37°C for 45 min. After the incubation period, the medium with the MTT solution was removed and then 100 µL of DMSO was added to dissolve the formazan crystals and the final product was placed in a 96-well plate. Finally, the absorbance was measured by a spectrophotometer at 492 nm. Each group’s response expressed as absorbance variation normalized as percent of control, considering absorbance of the positive cell control as 100% activity of culture, since those cells were not in coculture with PBMCs.
To ensure that MTT results reflected the metabolic activity of the EUC-MSCs, and that these measurements were not biased by remaining PBMC activity, an experiment comparing EUC-MSCs and PBMCs in coculture has been performed. This study had five conditions: (1) EUC-MSCs culture (basal control), (2) PBMC and EUC-MSCs coculture (test group), (3) EUC-MSCs cultured with 5% of DMSO (death control), (4) RPMI (MTT control), and (5) PBMC supernatant culture removed from the coculture in the MTT protocol assay (control group) [Figure 2].
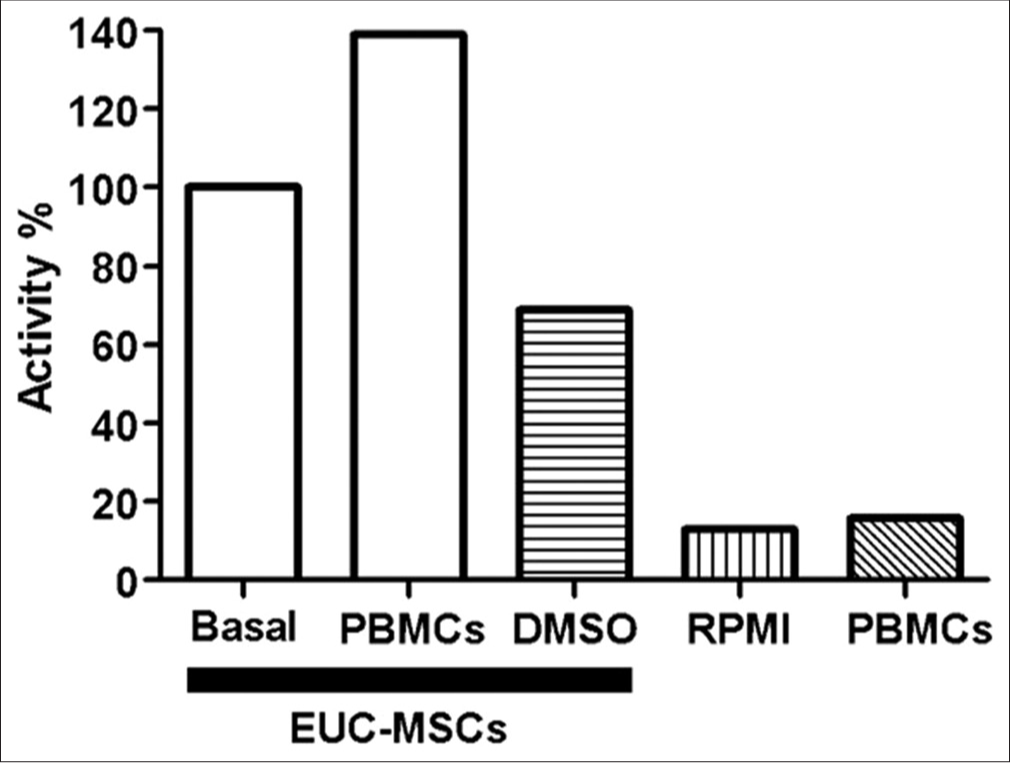
- Evaluation of PBMCs activity in EUC-MSCs coculture. (All conditions n=1). EUC-MSCs: Equine umbilical cord mesenchymal stem cells, PBMCs: Peripheral blood mononuclear cells.
Statistics analysis
The data were expressed in bar graphs as mean and standard error of the mean, and were statistically analyzed by the one-way ANOVA followed by the Dunnett’s multiple comparison test in which: P > 0.05 (ns), P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***). The Shapiro–Wilk test was employed to test the normality of the data distribution. Specifically, the percentage of activity of the treatment and placebo groups (between subjects) was compared with the repeated measures ANOVA; comparative EUC-MSC bar graphs, the percentage of activity of the groups at days 0, 14, 28, 42, and 56 was analyzed versus the pre-treatment activity by the within-subjects one-way ANOVA. The percentage of activity of DMSO was analyzed versus the baseline activity.
RESULTS
Cellular assay: MLR as an evaluation of the cellular response
Determination of DMSO percentage used as a negative/ death control
[Figure 1] shows the results of the DMSO cell toxicity curve obtained from four independent assays where at 5% of DMSO cell viability decreased by 35%, and thus, the concentration used in the “Death control” wells of the cellular response assay.
Evaluation of PBMCs activity in EUC-MSCs cocultures
The results obtained from PBMC supernatant culture [Figure 2], removed from the coculture in the MTT protocol assay, compared with the EUC-MSCs/PBMC coculture proved that the absorbance measured in the MTT assay was due to the EUC-MSCs metabolism, and not the PBMCs metabolism. The PBMCs supernatant removed in the MTT assay had no activity so that proved that all the PBMCs were removed from the coculture before performing the MTT assay in the EUC-MSCs. In conclusion, the MTT results of the cocultures were not biased by the PBMCs metabolic activity.
Repeated intra-articular administration of xenogeneic EUC-MSCs did not result in immunological response
To determine if repeated intra-articular administration of xenogeneic EUC-MSCs produced, or not, a cellular response, a cell-based assay (MTT tetrazolium assay) has been performed. PBMCs have been extracted from blood of placebo or treatment dogs on days 0, 14, 28, 42, and 56. PBMCs have been cocultured with their corresponding MSCs and, days after, an MTT assay has been performed, as described previously.
On day 0 (pre-treatment), PBMCs from treatment and placebo dogs significantly increased the activity of the EUCMSCs, with a EUC-MSCs death rate of 30% [Figure 3a].

- Metabolic activity of EUC-MSCs cocultured with their corresponding PBMCs of treatment and placebo dogs at different days. (a) Pre-treatment day (Basal n=8, Treatment n=8, Placebo n=8 and DMSO n=5), (b) day 14 (Basal n=5, Treatment n=8, Placebo n=8 and DMSO n=5), (c) day 28 (Basal n=7, Treatment n=8, Placebo n=8 and DMSO n=5), (d) day 42 (Basal n=7, Treatment n=8, Placebo n=8 and DMSO n=5), (e) day 56 (Basal n=7, Treatment n=8, Placebo n=8 and DMSO n=5). EUC-MSCs: Equine umbilical cord mesenchymal stem cells, PBMCs: Peripheral blood mononuclear cells, DMSO: Dimethyl sulfoxide.
Moreover, on day 14 post-first administration, the activity of EUC-MSCs in both treatment and placebo dogs was similar [Figure 3b]. The death rate of EUC-MSCs was 30%.
At day 28, post-first treatment and pre-second administration PBMCs of treated and placebo dogs had similar activity in EUC-MSCs [Figure 3c]. The death rate was 37% for EUCMSCs. Besides, at day 42 post-second administration [Figure 3d], there has been a significant increase in the activity of EUC-MSCs in placebo dogs. The death rate was 37% for EUC-MSCs.
Finally, at day 56, [Figure 3e], PBMCs of the treatment and placebo dogs have increased the activity of EUC-MSCs, but only placebo dogs had a significant increase. The death rate was 20% for EUC-MSCs.
All data have been represented in [Figure 4] and results show that PBMCs from treated and placebo dogs have maintained constant the metabolic activity of the EUC-MSCs during the entire trial and there has been a significant decrease in the death rate (30%).
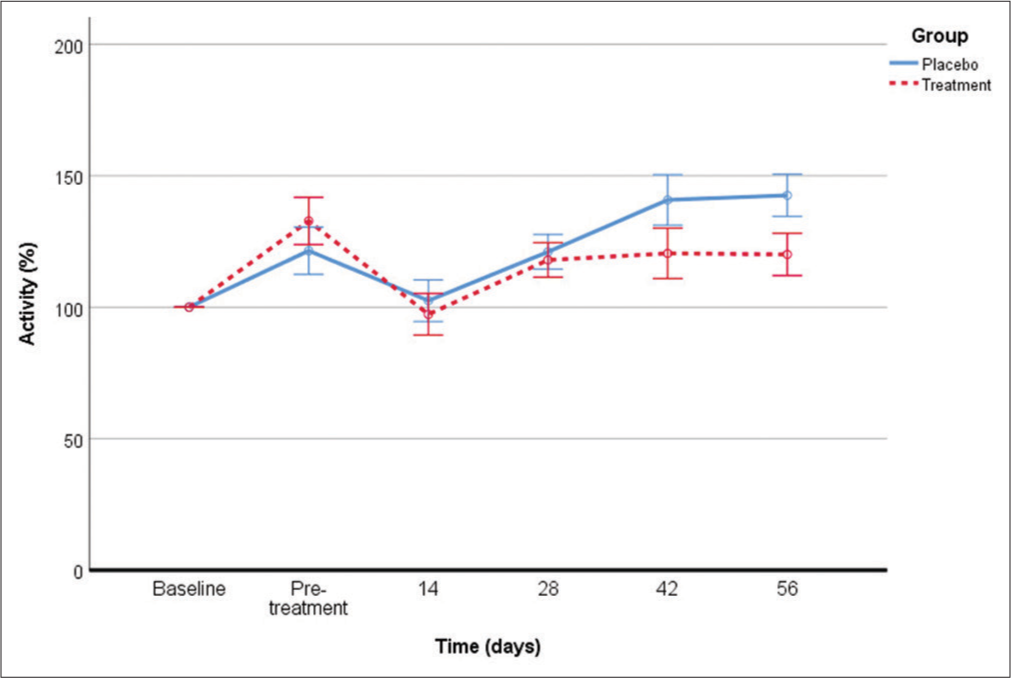
- Comparative of the metabolic activity during the entire trial of the EUC-MSCs cocultured with their corresponding PBMCs of treatment and placebo dog. (Basal n=7, DMSO n=25, treatment: Pre-treatment n=8, day 14 n=8, Day 28 n=8, day 42 n=8, day 56 n=8, placebo: Pre-treatment n=8, day 14 n=8, day 28 n=8, day 42 n=8, day 56 n=8). EUC-MSCs: Equine umbilical cord mesenchymal stem cells, PBMCs: Peripheral blood mononuclear cells, DMSO: Dimethyl sulfoxide.
DISCUSSION
In veterinary medicine, xenotransplantation could be a useful alternative to the costly and inconvenient auto and allogeneic transplantation. AD-MSC auto-transplantation treatment faces many challenges, including: (1) Veterinary practices lack the equipment and expertise for AD-MSC isolation, (2) excision of adipose tissue causes donor site morbidity, (3) individually AD-MSC isolation is costly and time consuming, and (4) at least two veterinarian appointments are needed for adipose tissue procurement and AD-MSC injection. However, these obstacles can be overcome when therapeutic MSCs are obtained from allogeneic or xenogeneic sources. For example, EUC-MSCs can be prepared in commercial scale quantities and stored in liquid nitrogen until shipped and injected into the diseased dog. Thus, there are no extra costs for the veterinarian or the owner.
The advantages of xenogeneic MSCs include the almost unlimited availability of cell numbers and immediate access. Disadvantages might include the risks of disease transmission from donor to recipient. Xenogeneic cell transplantation has been reported to be safe in several pre-clinical studies using, for example, human AD-MSCs by intravenous administration to treat experimentally induced atrial injury in dogs, with no changes in PBMCs composition of the recipient dogs indicating immunocompatibility.[13] Furthermore, porcine AD-MSCs did not cause adverse effects, such as inflammatory or allergic reactions, in dogs suffering from osteoarthritis.[10]
From an immunological point of view, allotransplantation is a better alternative to xenotransplantation. However, ethically, the harvest of canine adipose tissues for commercial purposes is a less acceptable alternative compared to that of equine umbilical tissues. Thus, xenotransplantation of EUCMSCs for veterinary patients can be considered a better option. Therefore, a Target Animal Safety study was required to determine if xenogeneic transplantation of EUC-MSCs to young healthy dogs might produce a cellular immune response.[22]
A relevant issue when administering EUC-MSCs is the administration pattern. It has been suggested that allogeneic transplantation of EUC-MSCs can be administered on at least two occasions without eliciting a measurable cellular immune response, but it is unknown whether an increased number of injections would produce such a response.[7] We found no decrease in the metabolic activity of EUC-MSCs after both treatments administered 4 weeks apart, suggesting a lack of cytotoxic effect following xenotransplantation with EUC-MSCs.
Overall, we observed that EUC-MSCs cells did not show cell activity when cocultured with PBMCs in comparison with the placebo results during 56 days of study. Thereby, these results suggest that PMBCs from the treated dogs administered with xenogeneic dose of EUC-MSCs do not generate a cytotoxic response, as no cytotoxic reaction occurs between lymphocytes and EUC-MSCs promoting cytotoxicity.
Moreover, a feasibility study for the xenogeneic use of equine stem cells from peripheral blood in six dogs suffering from OA demonstrated that after a single intraarticular administration of 2 × 106 cells, no changes were detected in blood test. The treatment was well tolerated and reduced pain and lameness as assessed through subjective measurements (owner questionnaires).[23] This study, with the limitation of one single administration, demonstrated that the use of equine stem cells in dogs is safe and could be an alternative to adipose extraction from the patient and can be applied with no delay as it could be accessible under request in a shorter time.[24]
Xenogeneic stem cell transplantation has, therefore, several advantages including potentially unlimited supply, lower cost, and quality control. The previous research studies have demonstrated that xenogeneic stem cell transplantation has relevant therapeutic effects in a variety of diseases such as liver failure and myocardial infarction but also chronic diseases such as advanced type 1 diabetes mellitus, myelosuppression, and other end-stage diseases based on the direct replacement of the dysfunctional cells.[25]
CONCLUSION
Xenogeneic stem cell transplantation of EUC-MSCs provides a new strategy for the treatment of chronic diseases like canine osteoarthritis although safety confirmation is required. This study, involving a repeated intra-articular administration of xenogeneic EUC-MSCs at a higher dose (7.5 × 106 cells), has demonstrated no cytotoxic changes of the immune system of the recipient dogs, potentially negatively affecting their viability. Overall these results suggest that the administration of EUC-MSCs can be considered as safe.
Acknowledgments
To Equicord for providing the data of this study to be included in the PhD of the corresponding author, to the Chief of the Centro Cinológico for supporting the study, to the Veterinary team of the Centro Cinológico and all the staff personnel involved in the development of the study, to Dr. Jose Luis Puchol and Dr. Miguel Angel Cabezas for their contribution in the development of the study performing the intra-articular administrations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The study was supported by Equicord S.L. as assessments included in a Target Animal Safety Study (TAS).
Conflicts of interests
The authors Elena Garcia-Pedraza and Ana Gallego de Miguel declare that during the study development they both worked for Equicord.
References
- Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluid-derived progenitor cells. Vet Res Commun. 2011;35:103-21.
- [CrossRef] [PubMed] [Google Scholar]
- Isolation of equine multipotent mesenchymal stromal cells by enzymatic tissue digestion or explant technique: Comparison of cellular properties. BMC Vet Res. 2013;9:221.
- [CrossRef] [PubMed] [Google Scholar]
- Preclinical studies with umbilical cord mesenchymal stromal cells in different animal models for muscular dystrophy. J Biomed Biotechnol. 2011;2011:715251.
- [CrossRef] [PubMed] [Google Scholar]
- Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy. 2017;19:784-97.
- [CrossRef] [PubMed] [Google Scholar]
- Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev. 2014;23:1258-65.
- [CrossRef] [PubMed] [Google Scholar]
- Wharton's jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692-712.
- [CrossRef] [PubMed] [Google Scholar]
- Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 2011;13:1180-92.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol. 2013;156:99-106.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet Q. 2014;34:92-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-articular transplantation of porcine adipose-derived stem cells for the treatment of canine osteoarthritis: A pilot study. World J Transplant. 2014;4:196-205.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet Res. 2014;10:143.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Comp Orthop Traumatol. 2013;26:453-60.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012;21:2770-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hip osteoarthritis in dogs: A randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int J Mol Sci. 2014;15:13437-60.
- [CrossRef] [PubMed] [Google Scholar]
- Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet J. 2013;195:98-106.
- [CrossRef] [PubMed] [Google Scholar]
- Transplantation of human adipose mesenchymal stem cells in non-immunosuppressed GRMD dogs is a safe procedure. Stem Cell Rev Rep. 2016;12:448-53.
- [CrossRef] [PubMed] [Google Scholar]
- Veterinary Medicines and Inspections. 2008. VICH Topic GL43 Guideline on Target Animal Safety for Veterinary Pharmaceutical Products Draft Agreed by Vich Steering Committee. Doc Ref. EMEA/CVMP/ VICH/393388/2006. London: European Medicines Agency; Available from: https://www.ema.europa.eu/en/vichgl43-target-animal-safety-pharmaceuticals [Last accessed on 2021 Aug 29]
- [Google Scholar]
- Veterinary Medicines and Inspections. 2021. VICH GL44 Target Animal Safety for Veterinary Live and Inactivated Vaccines. Amsterdam, Netherlands: European Medicines Agency; Available from: www.ema.europa.eu/en/vich-gl44-target-animal-safety-veterinary-live-inactivated-vaccines [Last accessed on 2021 Aug 29]
- [Google Scholar]
- Veterinary Medicines and Inspections, Buckingham. 2020. Stem Cell-Based Products for Veterinary Use: Specific Question on Target Animal Safety Addressed by CVMP/ADVENT. London: European Medicines Agency; Available from: https://www.ema.europa.eu/en/stem-cell-based-products-veterinary-use-specific-question-target-animal-safety-addressed-cvmpadvent [Last accessed on 2021 Aug 29]
- [Google Scholar]
- Veterinary Medicines and Inspections. 2000. Vich GL9 Good Clinical Practices Guidelines, 2000. London: European Medicines Agency; Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/vich-gl9-good-clinical-practices-step-7_en.pdf [Last accessed on 2021 Aug 31]
- [Google Scholar]
- Development of the short-form glasgow composite measure pain scale (CMPS-SF) and derivation of an analgesic intervention score. Anim Welf. 2007;16:97-104.
- [Google Scholar]
- Mesenchymal stromal cell immunology for efficient and safe treatment of osteoarthritis. Front Cell Dev Biol. 2020;8:567813.
- [CrossRef] [PubMed] [Google Scholar]
- A feasibility study on the use of equine chondrogenic induced mesenchymal stem cells as a treatment for natural occurring osteoarthritis in dogs. Stem Cells Int. 2019;2019:4587594.
- [CrossRef] [PubMed] [Google Scholar]
- Review: Mesenchymal stem cell therapy in canine osteoarthritis research: “Experientia docet” (experience will teach us) Front Vet Sci. 2021;8:668881.
- [CrossRef] [PubMed] [Google Scholar]
- Xenogeneic stem cell transplantation: Research progress and clinical prospects. World J Clin Cases. 2021;9:3826-37.
- [CrossRef] [PubMed] [Google Scholar]







