Translate this page into:
Pathological Findings of Feline Pansteatitis (Yellow Fat Disease) in a 9-Month-Old Siberian Queen

*Corresponding author: Abdulhakeem Binhambali, Department of Clinical Sciences, Tuskegee University, Tuskegee, United States. email2hambali@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hassan B, Enam SJ. Nuhu SM, Binhambali A, Mustapha RA, Saleh A, et al. Pathological Findings of Feline Pansteatitis (Yellow Fat Disease) in a 9-Month-Old Siberian Queen. Res Vet Sci Med. 2025;5:5. doi: 10.25259/RVSM_9_2024
Abstract
Feline pansteatitis (FP), also known as yellow fat disease, is an inflammatory condition affecting cats’ adipose tissue, often linked to imbalanced diets rich in unsaturated fatty acids and low in vitamin E. This case report describes a 9-month-old Siberian Queen presented with painful subcutaneous nodules, lethargy, and icterus. Clinical findings included neutrophilic leukocytosis (Bands: 0.44 × 109/L, Neutrophils: 17.10 × 109/L) and elevated liver enzymes (Alkaline phosphatase: 44.8 U/L, Alanine transaminase: 93.0 U/L), while ultrasonography revealed hyperechoic masses and subcutaneous fat necrosis on necropsy with ceroid pigment deposition in abdominal organs. The aim of the presented case report was to describe clinical and pathological findings in a case of FP. This is the first report of FP in feline species from Africa.
Keywords
Feline pansteatitis
Fat necrosis
Pathology
Feline
Necropsy
Diet
INTRODUCTION
Yellow fat disease, commonly referred to as feline pansteatitis (FP), is a serious inflammatory condition that affects cats’ adipose tissue. It is a nutritional disorder characterized by inflammation, necrosis, and deposition of a ceroid pigment in fat cells caused by an excessive dietary intake of polyunsaturated fatty acids as well as low vitamin E levels in diets (Kang et al., 2022; Steffl et al., 2020).[1,2] The disorder results from an imbalance arising either from insufficient intake of vitamin E in the diet or from the consumption of high levels of unsaturated fatty acids (UFAs) that deplete the vitamin E or both (Niza et al., 2003).[3] As UFAs are found in the lipids of cellular membranes and in dietary fats, they are highly susceptible to peroxidation. Vitamin E (tocopherol), a biological antioxidant, protects fat from oxidative damage. A disproportion between the intake of UFAs and vitamin E results in the formation of peroxides and hydroperoxides, which cause severe inflammation and necrosis of the body tissues (Niza et al., 2003; Tidholm et al., 1996).[3,4]
While the majority of FP cases recorded in literature link the disease to consuming fish, particularly oily fish such as tuna and sardines (Niza et al., 2003),[3] the condition has also been reported in cats fed diets that include beef, chicken, liver, and pig’s brain (Niza et al., 2003; Watson et al., 1973).[3,5] Food processing-induced vitamin E inactivation, rancidity of fat in diet as well as obesity, has also been demonstrated to predispose cats to the disease (Tidholm et al.,1996;Fabbrini et al., 2005).[4,6]
Fever, lethargy, inappetence, and pain on palpation of subcutaneous nodular masses are the primary clinical signs of FP, but as the condition progresses, affected cats become less agile, and mostly unwilling to move around, and exhibit hyperesthesia, particularly over the back and abdomen (Kang et al., 2022).[1] A diagnosis can be made based on clinical findings, particularly painful palpable nodular subcutaneous masses, ultrasonography (which reveals increased echogenicity of peritoneal structures), serum biochemistry, histology of biopsied masses, and exploratory celiotomy (which reveals yellowish ceroid deposition on the omentum, mesentery, and other abdominal organs) (Watson et al., 1973; Zini et al., 2007)[5,7] can reveal the underlying issues, however, tests for erythrocyte membrane integrity or plasma tocopherol levels can also provide additional confirmation (Niza et al., 2003)[3]. While supportive therapy and the administration of corticosteroids have shown to be helpful, the management of FP entails dietary modification and vitamin E supplementation (Niza et al., 2003; Tidholm et al., 1996; Koutinas et al., 1992).[3,4,8] Early treatment has a fair prognosis, but severe cases may have a poor or grave prognosis (Kang et al., 2022).[1]
Despite the increasing recognition of this disease in many parts of the world, there have been no reports of FP in Africa to the best of our knowledge. This paper describes the clinical, laboratory, and pathological findings in a cat with FP, as well as the relationship between the disease and unconventional fatty homemade meals.
CASE REPORT
A 9-month-old Siberian Queen of 7 kg was presented to the Small Animal Clinic Unit of the Veterinary Teaching Hospital of Ahmadu Bello University, Zaria, on February 3, 2022, with a primary complaint of multiple swellings in the ventral abdominal region. The swellings had been observed a month before presentation, initially presenting as three distinct masses. The owner mentioned that a previously owned queen died after exhibiting similar symptoms, and a tom mate succumbed a few days after undergoing surgery for similar swellings (they are all from the same parents). The patient had no previous history of medical or vaccination records and had been fed exclusively on fish (tuna), oily milk, and meat. The cat was admitted for clinical diagnosis and treatment.
Clinical findings and initial management
Upon physical examination, the cat presented with multiple palpable, painful subcutaneous nodular masses located in the thoracic, ventral abdomen, and hindlimb regions. Additional findings included slightly enlarged popliteal lymph nodes, depression, and reluctance to move. The patient’s vital parameters at presentation were not within the normal range with exception of respiratory rate that was within the range as presented in Table 1.
| Parameters | Day 0 | Day 1 | Day 2 | Day 3 | Reference range |
|---|---|---|---|---|---|
| Temperature (°C) | 41.5 | 38.4 | 38.4 | 36.8 | 38.1–39.2 |
| Pulse rate (beats/min) | 70 | 77 | 68 | ---- | 140–220 |
| Respiratory rate (cycle/min) | 30 | 18 | 20 | 24 | 20–42 |
-----: Pulse non-palpable
The cat was administered an intramuscular injection of Piroxicam (Jamin Int’l co. Ltd) at a dose of 0.3 mg/kg (total 1.2 mg) to manage pain and pyrexia pending further diagnostics. The vital parameters of the patients from the day of presentation to day 4 of hospitalization can be found in Table 1.
Laboratory diagnostics
Ultrasonography (Sonostar S10TM, Sonostar Technologies Company, Guangzhou, China) revealed an hyperechoic masses in the ventral abdomen, thoracic, and hindlimb regions, with associated hyperechogenicity of abdominal structures, suggesting the involvement of the abdominal cavity [Figure 1a]. Aspiration biopsy of the subcutaneous nodular masses revealed necrosis of the subcutaneous adipocytes [Figure 1b]. Clinical pathology results as shown in Table 2 revealed marked neutrophilic leukocytosis with a left shift, while serum biochemistry indicated elevated liver enzymes, bilirubin, and triglycerides, as well as increased in albumin. Despite supportive care during hospitalization, the patient passed away on the 4th day of hospitalization.
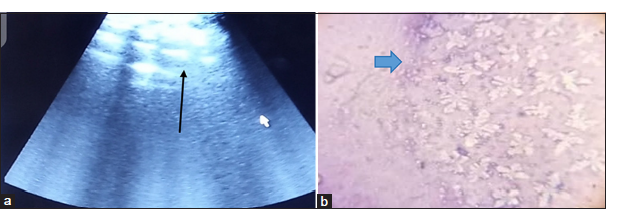
- (a) Ultrasonography of the ventral abdominal region of the patient showing hyperechoic nodular subcutaneous masses (black arrow). (b) Photomicrograph of the aspiration biopsy smear showing necrosis of the subcutaneous adipocytes (Giemsa ×100)
| Parameters | Cat’s values | Reference values |
|---|---|---|
| PCV (%) | 33.00 | 30.00–45.00 |
| Hb (g/dL) | 11.00 | 9.80–15.40 |
| TWBC (×109/L) | 22.00 | 5.50–19.50 |
| Bands (×109/L) | 0.44 | 0.00–0.30 |
| Neutrophils (×109/L) | 17.10 | 2.50–12.50 |
| Lymphocytes (×109/L) | 4.41 | 1.50–7.00 |
| Eosinophils (×109/L) | 0.00 | 0.00–0.80 |
| Monocytes (×109/L) | 0.00 | 0.00–0.90 |
| Basophils (×109/L) | 0.00 | 0.00–0.14 |
| Total plasma protein (g/dL) | 7.00 | 6.00–7.50 |
| Alkaline phosphatase (U/L) | 44.8 | 0.0–39.7 |
| Alanine transaminase (U/L) | 93.0 | 0.0–70.0 |
| Total bilirubin (μmoL/L) | 8.4 | <3.4 |
| Total protein (g/dL) | 53.8 | 54.7–78.0 |
| Albumin (g/dL) | 19.8 | 21.0–33.0 |
| Globulin (g/dL) | 34.0 | 26.0–51.0 |
| A/G ratio | 0.7 | 0.6–1.1 |
| Triglycerides (mg/dL) | 350.0 | 27.0–94.0 |
Hb: Hemoglobin, PCV: Packed cell volume, TWBC: Total white blood cell count, A/G: Albumin/Globulin ratio
Post-mortem (Gross) and histopathology findings
A post-mortem examination was conducted, revealing widespread pathological changes across multiple organ systems. The subcutaneous tissue exhibited yellowish fat deposition [Figure 2]. The lungs were congested, with areas of red hepatization [Figure 3a], and yellowish fat deposits were noted on the pericardium [Figure 3b], omentum, and mesentery and the lumen of the duodenum. In addition, there was generalized enlargement of the spleen, kidney, pancreas [Figure 4a], and liver [Figure 4b] with congestion and subcapsular necrotic hepatitis. There was intraperitoneal fluid (oily) of about 300 mL and yellow discoloration of the kidneys [Figure 4c], with fat deposits in the renal pelvis.
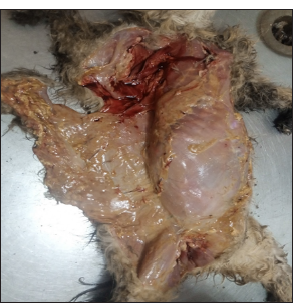
- Subcutaneous yellow fat deposition was revealed at the time of necropsy.
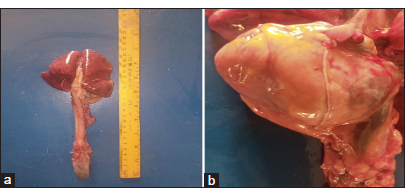
- (a) Congested lungs with areas of red hepatization, (b) Yellowish fat deposition on the pericardium.
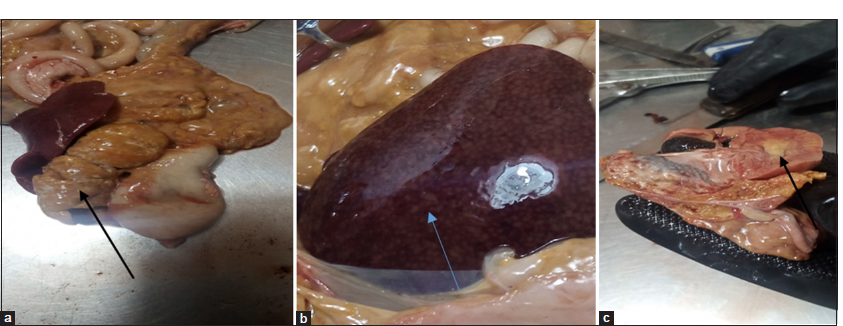
- (a) Markedly enlarged and yellowish discolored pancreas (black arrow). (b) The enlarged liver at necropsy showing congestion and widespread subcapsular necrotic foci (blue arrow). (c) Yellowish fat surrounding the kidney (black arrow) and fat deposits within the renal pelvis.
Histopathological examination of the pancreas, liver, and the kidney revealed extensive pathological changes. The pancreas showed necrosis and vacuolation of pancreatic cells, with fibrous connective tissue deposition and neutrophilic infiltration [Figure 5a]. The liver exhibited complete loss of hepatic architecture, widespread coagulative necrosis of hepatocytes, mononuclear cellular infiltration, and areas of hemorrhage [Figure 5b]. The kidney demonstrated widespread necrosis of renal tubular epithelial cells, with fat vacuoles in the lumen of the convoluted tubules and collecting ducts [Figure 5c]. These findings pointed to significant systemic involvement, likely contributing to the cat’s rapid deterioration and possible death during hospitalization.
![(a) Photomicrograph of the pancreas showing necrosis (blue arrows) and vacuolation (black arrows) of the pancreatic cells, with widespread fibrous connective tissue deposition (black asterisk) and mononuclear cell infiltration (H&E ×400). (b) Photomicrograph of the liver showing necrosis of hepatocytes and mononuclear cellular infiltration (hematoxylin and eosin [H&E] ×400) mononuclear cellular infiltration (red arrow), necrosis of hepatocytes (blue arrow) (c) Photomicrograph of the kidney showing widespread necrosis and vacuolation of renal tubular epithelial cells (blue arrows), with vacuoles in the lumen of the convoluted tubules and collecting ducts (red arrow).](/content/122/2025/5/1/img/RVSM-5-5-g005.png)
- (a) Photomicrograph of the pancreas showing necrosis (blue arrows) and vacuolation (black arrows) of the pancreatic cells, with widespread fibrous connective tissue deposition (black asterisk) and mononuclear cell infiltration (H&E ×400). (b) Photomicrograph of the liver showing necrosis of hepatocytes and mononuclear cellular infiltration (hematoxylin and eosin [H&E] ×400) mononuclear cellular infiltration (red arrow), necrosis of hepatocytes (blue arrow) (c) Photomicrograph of the kidney showing widespread necrosis and vacuolation of renal tubular epithelial cells (blue arrows), with vacuoles in the lumen of the convoluted tubules and collecting ducts (red arrow).
DISCUSSION
Management of FP typically involves dietary modification and vitamin E supplementation, with the success of these interventions heavily dependent on early disease recognition (Kang et al., 2022).[1] This case report illustrates how delays in diagnosis can lead to systemic complications, including widespread organ damage.
In the present case, we suspected that the homemade diet, primarily composed of fish and oily foods, contributed to the development of FP. This suspicion was further supported by the fact that previous siblings had experienced similar issues. Such a diet, lacking essential nutrients, likely played a pivotal role in triggering the disease. Oily fish, such as tuna and sardines, are well-documented sources of UFAs that are known to precipitate FP (Niza et al., 2003).[3] These findings emphasize the critical role of balanced nutrition in preventing such conditions, particularly when homemade diets are involved. Our results align with established research, which indicates that FP is typically triggered by an imbalance between unsaturated fatty acids (UFAs) and vitamin E intake, leading to oxidative stress in the form of peroxides and hydroperoxides, ultimately resulting in tissue inflammation and necrosis (Niza et al., 2003; Tidholm et al., 1996).[3,4]
The clinical signs observed in this cat including painful subcutaneous nodules, enlarged popliteal lymph nodes, elevated liver enzymes, bilirubin and triglycerides, and hypoalbuminemia, are all consistent with the classic presentation of the disease, as reported by Kang et al. (2022).[1] The fat necrosis and leukocytosis noted in this case were also described in one of the earliest documented cases of FP by Munson et al. (1958)[9] Ultrasonography revealed hyperechoic subcutaneous nodular masses, consistent with the characteristics noted by Kang et al. (2022) and Zini et al. (2007) and,[1,7] and biopsy results initially confirmed subcutaneous fat necrosis (panniculitis), a hallmark feature of FP, as reported by Watson et al. (1973) and Griffin (2021).[5,10] Furthermore, our gross findings of ceroid pigment deposition in the subcutaneous tissues correlated with the reports of Zini et al. (2007) and Koutinas et al. (1992),[7,8] in which similar cases were documented.
This case reveals the importance of balanced diets in preventing FP, particularly when homemade diets are utilized. Early diagnosis is a key to a favorable prognosis. Veterinarians must remain vigilant in recognizing clinical signs and implementing appropriate diagnostic and treatment protocols to reduce the risk of organ damage and improve outcomes, even though the prognosis remains guarded in cats exhibiting severe forms of the disease. In addition, this report also encourages the need for greater awareness among cat owners regarding the nutritional requirements of their pets. Veterinarians should encourage owners (especially in regions where knowledge of pet nutrition is limited) to consult veterinarians or veterinary nutritionists when preparing homemade diets to avoid nutritional imbalances that could lead to serious conditions such as FP.
CONCLUSION
We report a case of FP in a 9-month-old Siberian Queen, caused by an unbalanced homemade diet deficient in essential vitamins. Our findings are consistent with previous reports of FP from other parts of the world, and this case represents the first documented instance of FP reported from Africa.
Acknowledgment
The authors would like to thank the Head of the Department of Veterinary Medicine and the Director of the Veterinary Teaching Hospital at Ahmadu Bello University, Zaria, for their support and provision of necessary facilities. The authors also acknowledge the indispensable contributions of the veterinary technicians at the Department, whose dedication greatly contributed to the success of the study.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Tentative Diagnosis and Monitoring using Ultrasound in a Cat with Pansteatitis: A Case Report. Korean J Vet Res. 2022;62:e7.
- [CrossRef] [Google Scholar]
- Nodular Panniculitis in a Cat with High Alpha Tocopherol Concentration in Serum. Vet Med Sci. 2020;6:980-4.
- [CrossRef] [PubMed] [Google Scholar]
- Feline Pansteatitis Revisited: Hazard of Unbalanced Homemade Diets. J Feline Med Surg. 2003;5:271-7.
- [CrossRef] [PubMed] [Google Scholar]
- Feline Pansteatitis: A Report of Five Cases. Acta Vet Scand. 1996;37:213-7.
- [CrossRef] [PubMed] [Google Scholar]
- Feline Cutaneous and Visceral Necrotizing Panniculitis and Steatitis Associated with a Pancreatic Tumour. Vet Dermatol. 2005;16:413-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pansteatitis and Severe Hypocalcaemia in a Cat. J Feline Med Surg. 2007;9:168-71.
- [CrossRef] [PubMed] [Google Scholar]
- Pansteatitis (Steatitis, “Yellow Fat Disease”) in the Cat: A Review Article and Report of Four Spontaneous Cases. Vet Dermatol. 1992;3:101-6.
- [CrossRef] [Google Scholar]
- Steatitis (Yellow fat) in Cats feD Canned Red Tuna. J Am Vet Med Assoc. 1958;133:563-8.
- [Google Scholar]
- Feline Abdominal Ultrasonography: What's Normal? What's Abnormal?: Abdominal Lymph Nodes, Peritoneal Cavity and Aorta. J Feline Med Surg. 2021;23:835-49.
- [CrossRef] [PubMed] [Google Scholar]







