Translate this page into:
Surgical Management of Canine Transmissible Venereal Tumor with Adjunctive Chemotherapy Combination

*Corresponding author: Daniel Onimisi Avazi, Department of Veterinary Medicine, Surgery and Radiology, University of Jos/Veterinary Teaching Hospital, Jos, Nigeria. danooavaz@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Avazi DO, Alo-Aleje HO, Tanko PN, Galadima M. Surgical Management of Canine Transmissible Venereal Tumor with Adjunctive Chemotherapy Combination. Res Vet Sci Med. 2024;4:4. doi: 10.25259/RVSM_7_2024
Abstract
One male and a female 2-year-old Neapolitan Mastiff dogs weighing 41 and 40 kg, respectively, were presented to the University Veterinary Teaching Hospital, Jos, Nigeria, with complaints of continuous bleeding from the prepuce and vulva which were first observed about 2 months before presentation. Clinical examination revealed preputial bleeding of the male dog and serosanguinous vulva discharges with the presence of palpable masses with multiple nodules that were cauliflower-like. Cytological evaluations of smears from preputial and vaginal swabs revealed characteristic round cells with large eccentric nuclei, large nuclear-cytoplasmic ratio, and intracytoplasmic vacuolations as well as anisocytosis and anisokaryosis. Based on these cytological features, a diagnosis of canine transmissible venereal tumor (CTVT) was made in each case. Blood samples were collected for hematology and clinical chemistry. Debulking of the masses was carried out under general anesthesia using atropine sulfate and chlorpromazine as pre-anesthetics and Ketamine hydrochloride as induction and maintenance anesthetics. Following debulking, the resulting defect was reconstructed and the surgical wound was managed by daily wound cleaning and the administration of vincristine sulfate (0.025 mg/kg) slowly intravenously, ivermectin (0.3 mg/kg) subcutaneously, and piroxicam (0.3 mg/kg) and 20% amoxicillin (20 mg/kg) intramuscularly. There were no observed known vincristine sulfate-associated adverse effects as the dogs recovered and were discharged 3-week post-tumor debulking with no recurrence within 6 months of follow-up examinations. The debulked mass histologically showed several sheets of round neoplastic cells with prominent nuclei, mitotic figures, and infiltrating lymphocytes. Based on these findings, a diagnosis of CTVT was further confirmed. The hemogram showed moderate leukocytosis at day 1 in the bitch with a mild left shift at days 1 and 14 while clinical chemistry showed a marked increase in alkaline phosphatase, moderate increase in serum creatinine, and mild hypoproteinemia. In conclusion, management of CTVT with adjunctive vincristine sulfate and ivermectin combination resulted in complete healing with amelioration of known associated adverse effects.
Keywords
Canine transmissible venereal tumor
Vincristine sulfate
Ivermectin
Round cells
Wound
INTRODUCTION
A tumor is an abnormal mass of tissue whose growth exceeds and is uncoordinated with that of normal tissues and persists in the same excessive manner after cessation of the stimuli which evoked the change.[1] Tumors are a common problem in dogs with the paucity of information regarding the incidence, although estimates suggest that it is similar to humans, with at least one in ten dogs developing a tumor during her lifetime.[1,2] Canine transmissible venereal tumor (CTVT) is a round-cell tumor that generally affects the external genital organs of both male and female dogs.[2] CTVT is transmitted from one dog to another venereally by the physical implantation of viable tumor cells following coitus and/or social interactions with sniffing and leaking of the vulva region during the breeding seasons.[2,3] Although this tumor can occur at any age and in any breed and sex, it is frequently encountered in sexually active dogs. In male dogs, the tumor usually affects the cranial glans penis, prepuce mucosa, and bulb glands, while in female dogs, it is generally found in the caudal part of the vagina and the vulva.[2] They are usually benign, but there is a risk of relapse and metastasis to other organs, such as the skin, regional lymph nodes, tonsils, eyes, brain, pituitary, nose, tongue, lips, and mammary gland.[2,3] Signs include small pink-tored papules developing into nodules and multi-lobe papillae like cauliflower, which bleeds easily.[2,3] Diagnosis of CTVT is based on clinical signs cytological and histopathological examination. Microscopically, cells appear round, polyhedral, or slightly oval with indistinct cytoplasmic boundaries, multiple cytoplasmic vacuoles, and large foamy eccentric nuclei.[2] Chemotherapy with vincristine sulfate alone has proven very effective in the past, but its widespread abuse is most probably responsible for the frequent sub-optimal therapeutic effect and resistance currently encountered. This resistance is common due to inference from the overexpression of P-glycoprotein in various tissues which carry drugs into the extracellular medium.[2] Other management options include surgery, radiotherapy, and combination therapy.[2,3] Ivermectin works as a P-glycoprotein inhibitor by serving as a P-glycoprotein substrate for metabolism and this reduces the amount of these molecules in tissues.[2] Therefore, the adjunctive use of ivermectin in combination with vincristine sulfate in the management of CTVT proves beneficial as it prevents the immunosuppression associated with progressive lymphopenia produced by the sole use of vincristine.[2]
CASE REPORT
History and clinical examination
Two 2-year-old Neapolitan Mastiffs (a dog and a bitch) weighing 41 and 40 kg, respectively, were presented to the University Veterinary Teaching Hospital, Jos, Nigeria, with complaints of blood dripping from the prepuce and vulva. History revealed that this was first observed about a month after mating the bitch with the dog 2 months earlier. Clinical examination revealed pinkish-colored mucous membranes, normal vital parameters of temperature (37.5 and 38.4°C), pulse rate (65 and 69 beats/min), and respiratory rate (20 and 30 cycles/min), respectively, for the dog and bitch. Also observed were serosanguinous vulva and preputial discharges with the presence of palpable masses that were cauliflower-like in appearance [Figure 1]. Cytological evaluations revealed characteristic round cells with large eccentric nuclei, large nuclear-cytoplasmic ratio, and intracytoplasmic vacuolations as well as anisocytosis and anisokaryosis [Figure 2].
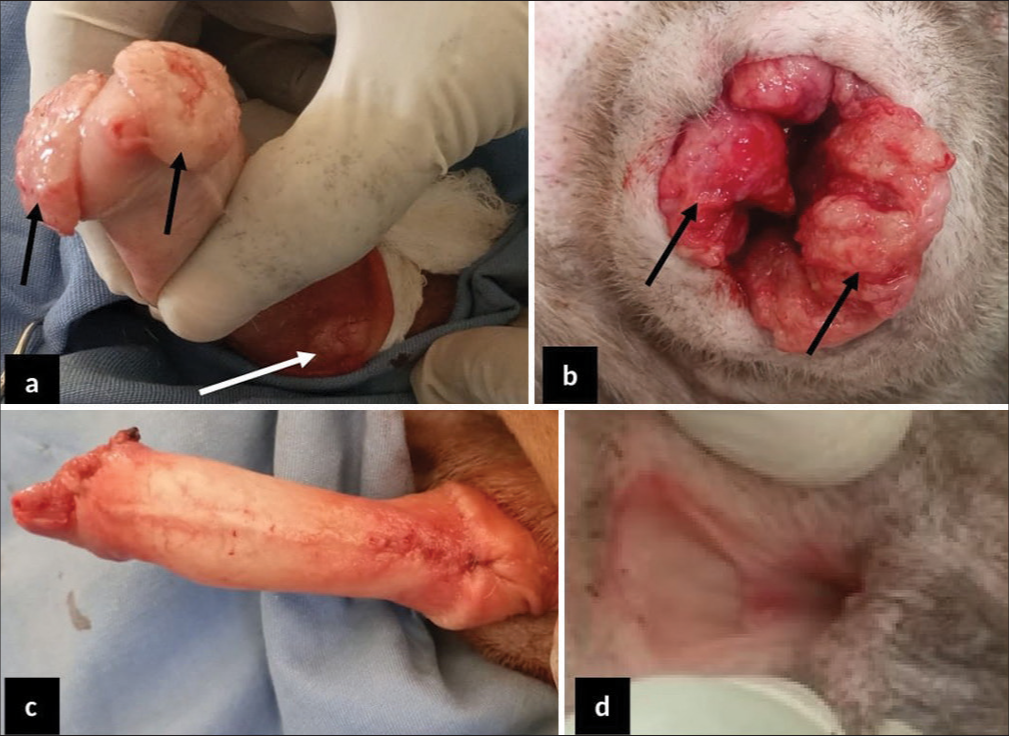
- 2-year-old male and female Neapolitan Mastiff dogs presented with cauliflower-like canine transmissible venereal tumor (CTVT). (a) The CTVT mass on the cranial glans penis (black arrow) and around the bulb glands (white arrow). (b) Cauliflower-like CTVT masses all over the vulva opening (black arrow). (c) The penis following debulking and defect reconstruction. (d) The fully healed vulva four weeks after management.
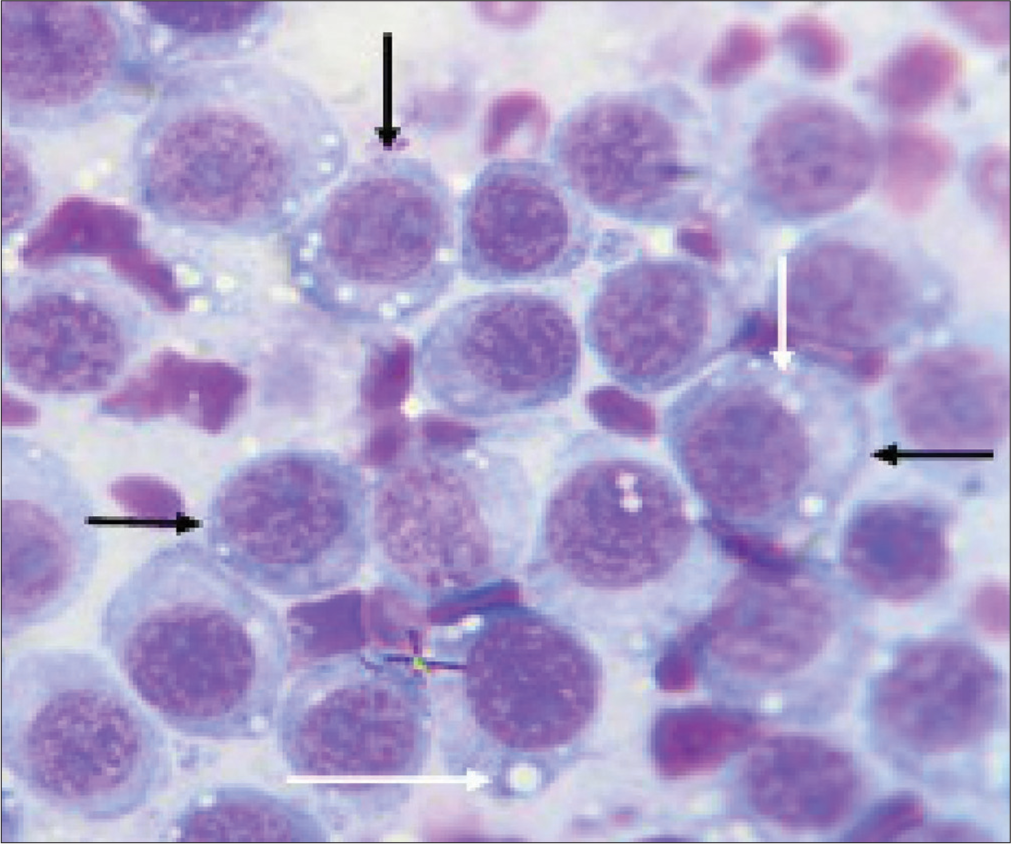
- Cytomorphology of canine transmissible venereal tumor in 2-year-old Neapolitan Mastiff dog. Showing; characteristic round cells having large eccentric nuclei (black arrows) and intracytoplasmic vacuolations (white arrows). Anisocytosis and anisokaryosis and a large nuclear-cytoplasmic ratio were noted. mag. ×1000.
Management
Based on history and clinical examination, a diagnosis of CTVT was reached. Surgical debulking of the masses was carried out under general anesthesia using atropine sulfate (Atropine® - Shanxi Shuguang Pharmaceutical Co., Ltd., Qixian, China) at 0.04 mg/kg, and chlorpromazine hydrochloride (Salvavidas Pharmaceutical Pvt. Ltd., Surat, India) at 4 mg/kg, intravenously as pre-anesthetics and Ketamine hydrochloride (Jawa ketamine® - Swiss Parenterals Ltd., Gujarat, India) at 11 mg/kg, intravenously as induction and maintenance anesthetics following aseptic surgical preparation of the patients. The masses were completely excised with a 3 mm circumferential margin of healthy tissues using a scalpel blade size 23. The debulked masses were then submerged in 10% neutral-buffered formalin for histological evaluation at the Pathology Unit of the University of Jos Veterinary Teaching Hospital [Figure 3].
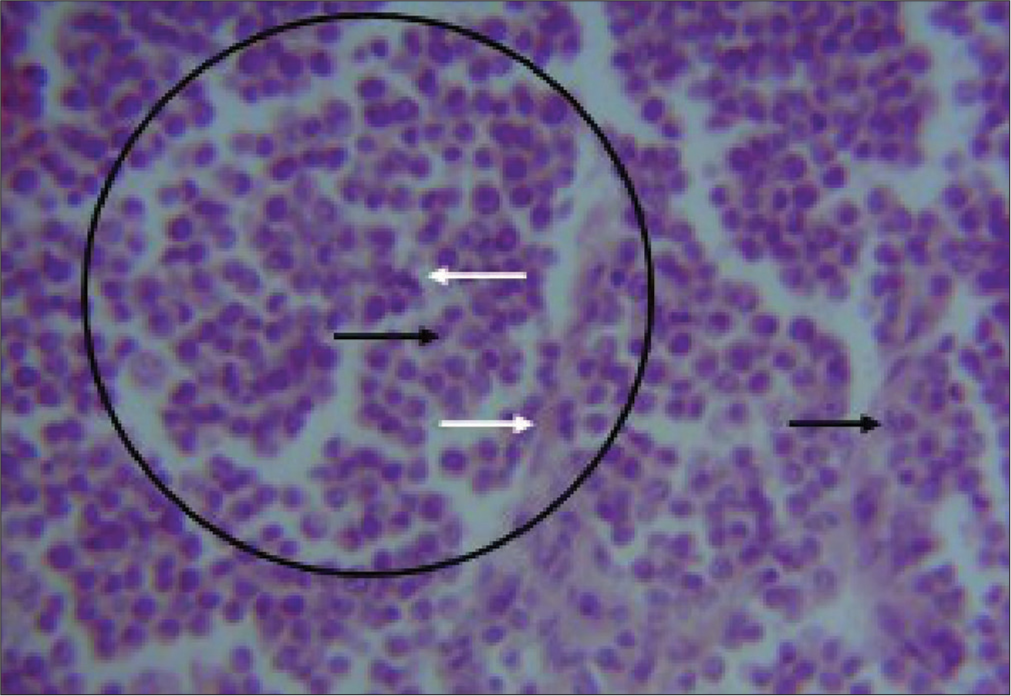
- Photomicrograph of canine transmissible venereal tumor in 2-year-old Neapolitan Mastiff dog. Shows; sheets of round neoplastic cells with prominent nuclei (black circled area) few mitotic figures (black arrow), surrounding collagen deposits (white arrows), and infiltrating lymphocytes. H&E Stain ×400.
Following debulking, the resulting defects were repaired by primary intention closure and the surgical wound was managed by daily wound cleaning and the administration of vincristine sulfate (Vinlon® Celon Laboratories Ltd., Telangana, India) at 0.025 mg/kg slowly intravenously ϰ4/52 once weekly and ivermectin (IVERMEC SUPER® - Pharma Swede, Egypt) at 0.3 mg/kg subcutaneously ϰ4/52 once weekly, piroxicam (Yuventis Pharmaceuticals, Baddi, India) at 0.3 mg/kg ϰ2/7 Q24 h intramuscularly, and 20% amoxicillin (Biocillin® InterChemie, La Waalre, Netherlands) at 20 mg/kg ϰ2/7 Q24 h intramuscularly. There were no observed known vincristine sulfate-associated adverse effects as the dogs recovered and were discharged after 3 weeks with no recurrence observed or reported for the 6-month follow-up appointments. A hemogram [Table 1] of the anticoagulated whole blood was obtained using the automated hematologic analyzer (Mindray® BC-3600, Shenzhen, China) and serum chemistry analytes such as alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatases (ALP), blood urea, creatinine, and total protein levels were measured by enzyme immunoassay method and read using digital ultraviolet spectrophotometer (Bio Tek Instruments Inc., Winooski, USA) [Figures 4-6].[4] The fixed tissue samples were routinely processed and stained with hematoxylin and eosin (H and E).[5] The slides were then examined under a light microscope (Olympus Research microscope, CH20i binocular version, Shinjuku, Tokyo, Japan, with AmScope 10MP USB microscope digital camera + calibration kit, Mainland, China) with objective 100ϰ and 400ϰ. Histopathological examination revealed several sheets of round neoplastic cells with prominent nuclei, mitotic figures, and infiltrating lymphocytes [Figure 3].
| Time | Day 1 | Day 7 | Day 14 | Day 21 | ||||
|---|---|---|---|---|---|---|---|---|
| Parameters/animal | Dog | Bitch | Dog | Bitch | Dog | Bitch | Dog | Bitch |
| PCV (%) | 43.93 | 39.00 | 48.10 | 38.40 | 55.63 | 36.67 | 53.00 | 36.57 |
| HGB (g/dL) | 14.63 | 13.00 | 16.13 | 12.77 | 18.53 | 12.23 | 17.7 | 12.20 |
| WBC (ϰ109/L) | 11.50 | 20.97 | 14.31 | 13.60 | 10.14 | 10.87 | 12.50 | 10.33 |
| sNEUT (ϰ109/L) | 9.57 | 17.40 | 11.34 | 10.76 | 8.00 | 8.07 | 8.11 | 6.19 |
| bNEUT (ϰ109/L) | 0.16 | 1.19 | 0.38 | 0.34 | 0.04 | 1.05 | 0.19 | 0.35 |
| LYMPH (ϰ109/L) | 1.35 | 1.64 | 1.21 | 1.63 | 1.77 | 1.21 | 3.09 | 3.21 |
| MONO (ϰ109/L) | 0.04 | 0.48 | 0.50 | 0.34 | 0.10 | 0.46 | 0.23 | 0.36 |
| EOSIN (ϰ109/L) | 0.38 | 0.23 | 0.88 | 0.51 | 0.23 | 0.25 | 0.88 | 0.25 |
| TP (g/dL) | 6.90 | 7.33 | 6.60 | 7.20 | 7.43 | 6.63 | 7.93 | 7.33 |
Dog=2-year-old male Neapolitan Mastiff dog and Bitch=2-year-old female Neapolitan Mastiff dog. PCV: Packed cell volume, HGB: Hemoglobin concentration, WBC: White blood cell, sNEUT: Segmented neutrophil, bNEUT: Band neutrophil, LYMPH: Lymphocytes, MONO: Monocyte, EOSIN: Eosinophil, TP: Total protein, L: Liter
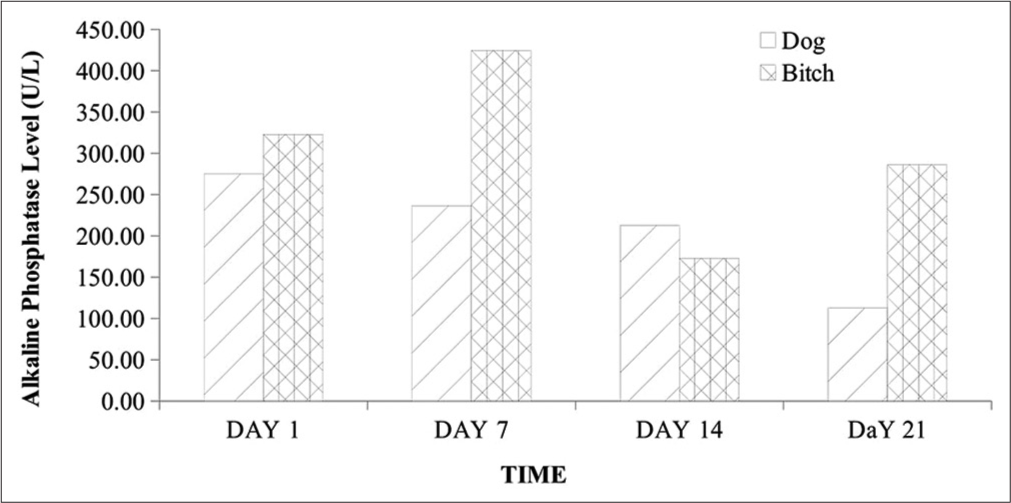
- Alkaline phosphatase level in 2-year-old male and female Neapolitan Mastiff dogs. U/L: Unit per liter.
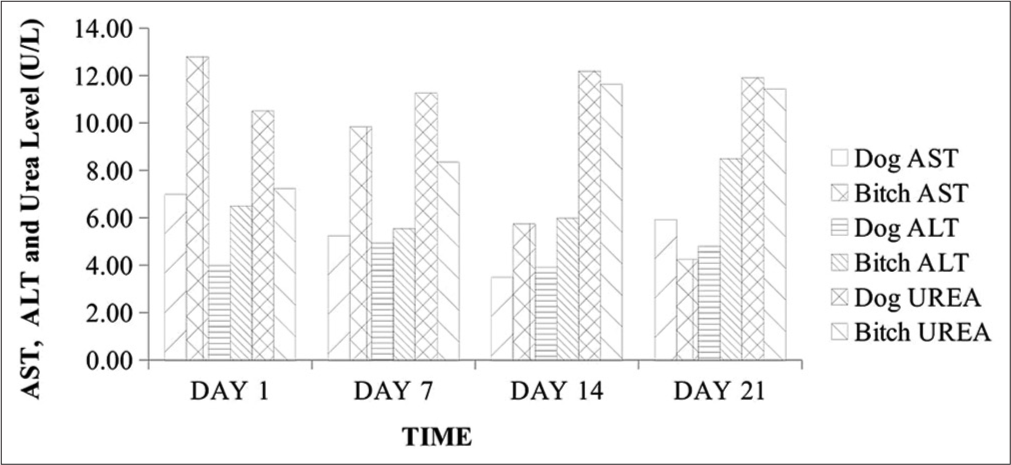
- AST, ALT, and urea levels in 2-year-old male and female Neapolitan Mastiff dogs. AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, U/L: Unit per liter.
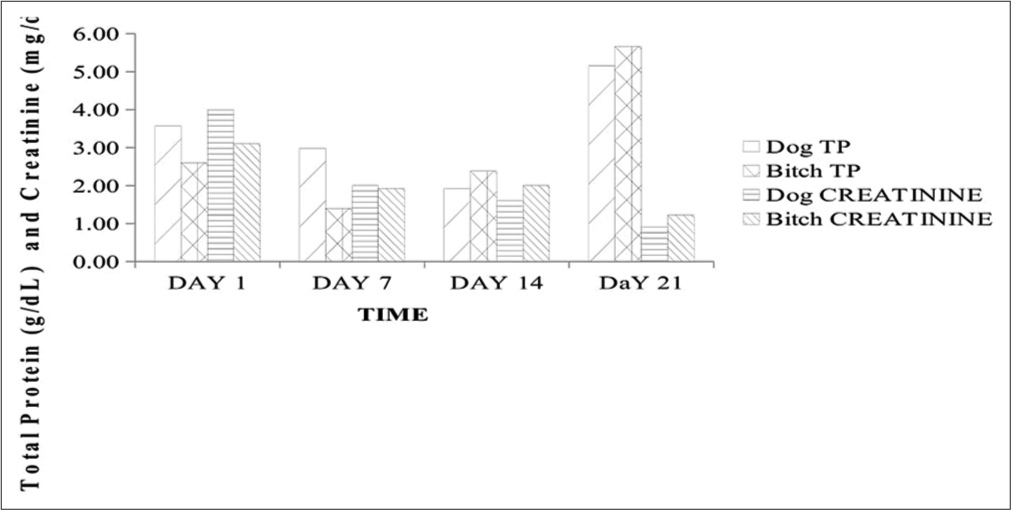
- Total protein and creatinine concentrations in 2-year-old male and female Neapolitan Mastiff dogs. g/dL: gram/decilitre, mg/dL: milligram per deciliter, TP: Total protein.
DISCUSSION
The history, clinical signs, gross, and cyto- and pathohistological morphology with visible characteristic round neoplastic cells were confirmatory for CTVT in these cases. The packed cell volume and hemoglobin concentration were largely within normal reference ranges throughout the management period despite the associated bleeding, although with a rise in values following management. This was consistent with a few previous reports of normal hemoglobin, hematocrit, and total erythrocyte count in CTVT and contrasted by Kabuusu et al. in which anemia resulted due to continuous bleeding.[3] Total WBC and neutrophil counts were slightly elevated in the bitch at presentation, which subsequently declined with fluctuations in values within normal reference limits alongside the dog throughout the management period. This initial elevation is probably due to hormonal effects on the inflammatory responses elicited by the progression of this tumor.[6,7] The fluctuation in WBC counts within normal reference limits is possibly due to the balance between the lymphocytes proliferating effects of ivermectin and the immunosuppressive effects of vincristine.[2,8] Studies have also shown that ivermectin administration is associated with an increase in the number of circulating CD4+ T cells with an increase in cellular-mediated immunity.[8] The band neutrophil, lymphocyte, eosinophil, and monocyte counts were not significantly altered as they fluctuated within the normal reference ranges throughout the management. These findings corroborate the reports by Kabuusu et al.,[3] in which they obtained normal counts of band neutrophils, eosinophils, and monocytes. The markedly elevated serum ALP levels could be linked to cytotoxicity with a significant release of this enzyme following vincristine sulfate deposition in the liver for detoxification.[9] Although AST and serum urea levels were normal, there was a fluctuating decrease in ALT level below the normal range limit and this is likely due to the cytotoxic effect of vincristine sulfate on hepatocytes leading to impaired hepatic function.[3] The serum creatinine levels were elevated with mild hypoproteinemia on day 1 but fluctuated subsequently with a decrease to normal reference limits by day 21. These findings could be ascribed to transient renal dysfunctions which are side effects of chemotherapy.[4,10]
In this study, we observed the complete remission of CTVT with adjuvant chemotherapy using a vincristine sulfate-ivermectin combination and a balance between the immunosuppressive effect of vincristine sulfate and lymphoproliferative effects of ivermectin. These corroborate the reports on the immunomodulatory effects of ivermectin with associated enhanced function of T-lymphocytes.[2,8]
CONCLUSION
In conclusion, management of CTVT with adjunctive vincristine sulfate and ivermectin combination resulted in complete healing, with amelioration of known associated adverse effects of inappetence, hair loss, anemia, and immunosuppression among others. Therefore, adjuvant chemotherapy with a vincristine sulfate-ivermectin combination should be adopted for the management of CTVT. In addition, the immunomodulatory properties of ivermectin may provide an alternative approach for treating other disease conditions associated with immunosuppression.
Acknowledgments
The author appreciates the immense contributions of Mr. James G and Mr. Abdulkareem YI for assisting with the laboratory analysis.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Vincristine and Ivermectin Combination Chemotherapy in Dogs with Natural Transmissible Venereal Tumor of Different Cyto-morphological Patterns : A Prospective Outcome Evaluation Vincristine and Ivermectin Combination Chemotherapy in Dogs with Natural Transmissible Venereal Tumor of Different Cyto-morphological Patterns : A Prospective Outcome Evaluation. Anim Reprod Sci. 2021;216:106358.
- [CrossRef] [Google Scholar]
- Clinicopathological Changes during Canine Transmissible Venereal Tumor Treatment with Vincristine. Indian J Vet Pathol. 2019;43:132-5.
- [CrossRef] [Google Scholar]
- Identification of Key Proteins in the Signaling Crossroads between Wound Healing and Cancer Hallmark Phenotypes. Sci Rep. 2021;11:17245.
- [CrossRef] [Google Scholar]
- Histopathological and Cytological Analysis of Transmissible Venereal Tumor in Dogs after Two Treatment Protocols. Colloq Agrar. 2012;8:36-45.
- [CrossRef] [Google Scholar]
- Evaluation of Levels of Interleukin-6, Interleukin-8 and Some Haematologic Parameters of Dogs with Cutaneous Wounds. Cytokine. 2019;113:128-38.
- [CrossRef] [Google Scholar]
- The Role of Neutrophils in the Immune System: An Overview. Methods Mol Biol. 2014;1124:3-10.
- [CrossRef] [Google Scholar]
- Ivermectin Converts Cold Tumors Hot and Synergizes with Immune Checkpoint Blockade for Treatment of Breast Cancer. NPJ Breast Cancer. 2021;7:22.
- [CrossRef] [Google Scholar]
- Effect of Vincristine Chemotherapy in TVT Affected Dogs. J Pharm Innov. 2018;7:163-6.
- [Google Scholar]
- A Review on Canine Transmissible Venereal Tumourfrom Morphologic to Biochemical and Molecular Diagnosis. AJAD. 2015;4:185-95.
- [Google Scholar]







