Translate this page into:
A Systematic Review based on the Use of Au- and Pt-based Nanoparticles along with H2 Blocker Medicines

*Corresponding author: Anupama Sharma, Department of Biochemistry, School of Medical and Allied Sciences, Sanskriti University, Mathura, Uttar Pradesh, India. shanuanupama@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma GC, Sharma A. A systematic review based on the use of Au- and Pt-based nanoparticles along with H2 blocker medicines. Res Vet Sci Med 2022;2:5.
Abstract
This study is a review and a cumulative piece of work on Pt- and Au-based nanoparticles and H2 blockers drugs. It has been studied that Pt- and Au-based nanoparticles are used more often in different biomedical applications. Nanoparticles based on these two metals are highly biocompatible and can be used for the diagnosis of various diseases through in vitro imaging, tissue engineering, and drug delivery. The compatibility of Pt- and Au-based nanoparticles is increased using polymer-nanocomposite hybrids, which can participate in delivering a large number of drugs to modulate and improve the biodistribution and uptake of drugs on the cellular level. Another part of this review focuses on H2 blocker drugs. These drugs are specific classes for the suppression of gastric juice or acids under different gastrointestinal conditions. Famotidine has also been reported as a marker drug for COVID-19 patients. This review provides cumulative information on H2 blocker drugs and nanoparticles in single articles and specifies their role in different treatments associated with different types of cancers, gastrointestinal disorders, and COVID-19.
Keywords
Gold nanoparticles
Platinum-based nanoparticles
H2 blocker drugs
Magnetic resonance imaging
Meta-analysis
INTRODUCTION
H2 blockers or antagonists are histamine blockers that are included in the class of agents specific for suppressing gastric acid in different gastric conditions. These drugs are approved by Food and Drug Administration (FDA) to be used for a short duration to treat gastroesophageal reflux diseases, ulcers of the duodenum, hypersecretion of gastric juice, indigestion, and infrequent heartburn. RA is also used to treat esophagitis, prophylaxis, urticaria, and gastrointestinal hemorrhage. H2 blockers are included in multidrug establishment for the eradication of Helicobacter pylori.[1] H2 blocker drugs can be also used during pregnancy as secondary medicine, although for heartburn during pregnancy antacids are preferred.[2] H2 Receptor Antagonist (H2RA) is also considered safe for adolescent children with mild heartburn issues.[3] The overall use of H2 blockers depends on the rigorousness of gastric illness therapy duration and dosage regimen. H2 blockers such as nizatidine, cimetidine, and famotidine are used more frequently. The mechanism of the action of H2 blockers depends on the reverse binding of the histamine in the parietal cells of the digestive system as they limit endogenous ligands and also inhibit their binding mechanism. Hence, an H2 blocker works as a good antagonist. Usually, gastric acid is released in the stomach after the meal when the histamine gets stimulated and releases the enterochromaffin cells which bind with H2 receptors.[4] When the intracellular cyclic Adenosine Monophosphate (cAMP) level is increased in the gastric cells by adenylate cyclase activation, more gastric acid is released. This cAMP activates protein kinase A which is involved in the movement of K+/H+ ATPase transporter through the plasma membrane, increased K+/H+ Adenosine Tri Phosphatase (ATPase) transport secretes more gastric acid in the parietal cells.[5] Acid secretion of the parietal cells is stimulated when the histamine receptors are blocked.[5] H2 blockers have the capacity of gastric relief from 1 h to 5 h by decreasing the secretion of gastric juices, it is similar to the onset of gastric stroke.[6]
Due to the wide therapeutic index of H2 blocker, toxicity is quite rare. It can have associated with the blocking of H2 receptors in case of myocardium issues and Central Nervous System (CNS) diseases such as depression, bradycardia, and hypotensive. These receptors are involved in rapid fusion veins of H2 RA. The treatment related to H2 RA toxicity requires decontamination of the gastric lavage or the use of activated charcoal with supportive care and discontinuation of the drugs.
TYPES OF H2 BLOCKER DRUGS STUDIED
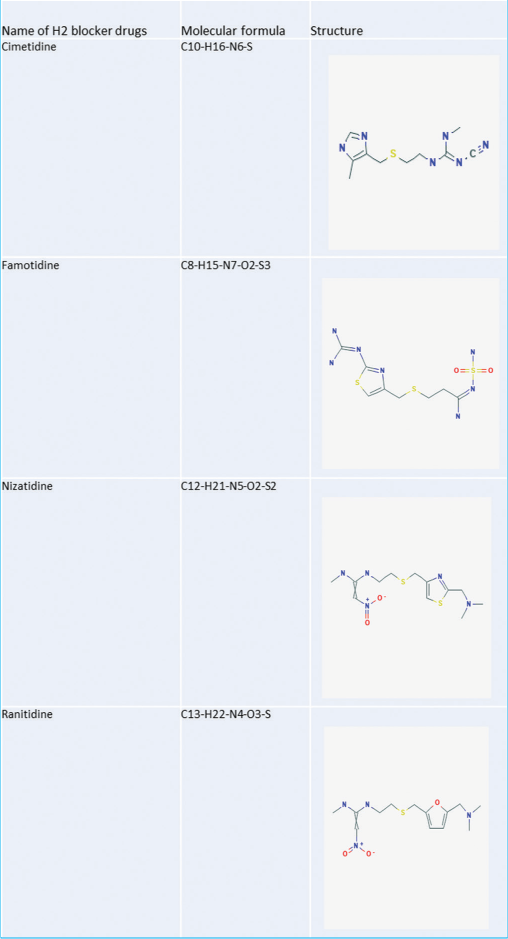
H2 blockers are categorized basically into four types; ranitidine, nizatidine, famotidine, and cimetidine [Table 1]. These H2 blockers are divided based on their ring structure [Table 2]. Two types of rings are seen, namely, the five-membered ring and the six-membered ring. In the present study, we emphasize two often used H2 blocker drugs famotidine and nizatidine.
| Name of H2 blocker drugs | Generic names | Strength 1–10 on scale | Difference in the metabolism | |
|---|---|---|---|---|
| Zantac | Ranitidine | 8 | Ranitidine is metabolized up to three metabolic cycles in the liver, 70% of the ranitidine is excreted out in the urine because it cannot be metabolized. | |
| Pepcid | Famotidine | 6.5 | Famotidine has thiazole nucleus and it is 7.4 times potent than ranitidine and it is 20 times potent with an equimolar concentration of cimetidine | |
| Axid AR | Nizatidine | 10 | 30% of the nizatidine is metabolized in four metabolic cycles, and 60% of the total nizatidine taken is active similar to its parent compound. About 7% of the nizatidine is converted into N2-monodesmethylnizatidine. | |
| Tagamet HB | Cimetidine | 9 | ||
| Pepcid complete | Famotidine, calcium carbonate and magnesium hydroxide | 8 | 30–35% of famotidine gets metabolized in the liver to S-oxide, rest is excreted out. | |
| Fluxid | Famotidine | Not available | ||
| Leader heartburn relief | Cimetidine | Not available | ||
| Equalize heartburn relief | Famotidine | Not available | ||
| Complete acid controller | Famotidine, calcium carbonate and magnesium hydroxide | 8 | ||
| Acid reducer | Ranitidine | Not available | ||
| Acid reducer non-prescription strength | Ranitidine | Not available | ||
| Axid oral solution | Nizatidine | Not available | ||
| Berkley and Jenson acid reducer maximum strength | Ranitidine | 10 | ||
|
Famotidine
Famotidine is an H2 blocker drug that decreases the acid production in the stomach to treat gastrointestinal conditions due to more secretion of the acid in the stomach. It blocks the H2 receptors situated on the parietal linings of the wall of the stomach. Histamine stimulates the parietal cells to release gastric acid. Famotidine blocks the H2 receptor; hence, the production of gastric acid is also reduced. Famotidine is an H2 blocker drug that is used to block the H2 receptors. These H2 receptors are responsible for allergic reactions. Famotidine is a drug that can be used for the short-term treatment of duodenal ulcers. It reveals the interaction with other drugs such as antifungal drugs such as ketoconazole, fluconazole, itraconazole, and anticoagulants such as aspirin, drugs for cancer such as dabrafenib, bosutinib, erlotinib, and pazopanib, drugs for epilepsy such as fosphenytoin, carbamazepine, phenobarbital, and fentanyl, drugs for hepatitis such as ledipasvir, telaprevir, boceprevir, and sofosbuvir, and drugs for Human Influenza Virus (HIV) such as indinavir, atazanavir, saquinavir, iron supplements, metformin, multivitamins, and loperamide.
Nizatidine
Nizatidine is also an H2 blocker drug that has been approved for being used as a disease for peptic ulcers. Nizatidine is a substitute for thiazole; it is structurally related to famotidine and chemically diverse from prototypical H2 blockers. Among all the available H2 blocker drugs, the most potent is famotidine, and the least effective is cimetidine whereas nizatidine is widely used and considered a safe drug for the treatment of peptic ulcers. Thiazole is a drug that is closely related to famotidine.[7] A major difference between these H2 blocker drugs is based on their bioavailability or administration potency, these drugs are effective when administrated in fewer amounts. The most potent difference among these H2 blocker drugs is their interaction with drugs and their hepatic oxidation.[8,9] Nizatidine binds with the microsomes of the human liver, whereas it has been seen in several studies that famotidine does not alter the kinetics of deposition of theophylline on a single dose in the hepatic cells.[10,11]
ROLE OF H2 BLOCKER PUMP IN COVID-19
Histamines are released from mast cells which further stimulate the H2 receptors for lung infection in the case of COVID-19 patients.[12] In COVID-19-infected cells, expression of the toll-like receptor is increased by the histamine, secreted from the mast cells, and Toll Like Receptor 3 (TLR3)-dependent signaling is reduced.[13] The bactericidal action is also increased, which further increases phagocytosis and suppresses the production of peroxide. Natural Killer (NK) cell count and cytotoxicity are increased by the production of peroxide ions, which results in enhanced Cluster of Differentiation 40 (CD40), Major Histocompatibility Complex 1 (MHC 1), Interleukin 2 (IL 2), and function of dendritic cells.[14] It has been reported that the use of famotidine an H2 blocker is found effective in the treatment of patients suffering from COVID-19. Mather et al., 2020, reported a study where a total of 878 patients received famotidine in COVID-19 infection and were cured successively.[15] They also reported low serum markers and the use of Cardio Pulmonary Resuscitation (CPR) in patients who received famotidine. A study published by Sun et al., 2021, also reported a better cure for COVID-19 in 36,635 patients receiving famotidine.[16]
NANOPARTICLES
The birth of nanotechnology has been considered in existence after the famous lecture of Richard Feynman an American physicist in 1959. Nanotechnology has emerged its arms in almost every field either medicine, science, agriculture, or engineering. Inorganic and organic hybrid materials are utilized in the field of nanotechnology. Metals such as silver and copper are used by humankind due to their antimicrobial properties. Resonance properties of the plasmon such as Ag NPs are found in calorimetric sensors such as fluorescence sensors, chemiluminescence sensors, and Raman spectrometry.[17] Nanotechnology develops at different levels such as devices, materials, and systems. In the present era, the most innovative study material is nanoparticles. These nanoparticles are microparticles or minor objects which are the complete unit and transport material. Nanoparticles are characterized based on their sizes such as diameter. The diameter of these particles ranges between 100 and 2500 nanometers, whereas the ultrafine particles range between 1 and 100 nanometers, this size is similar to ultrafine particles. The size-associated properties can or cannot be revealed by the nanoparticles, because properties in their nano form may vary from that in their bulk form.[18] Thus, reduction in the size of the particles from bulk to nano makes a substantial difference in their properties. The nanoparticles can be polymer-based, metallic, or mineral or they can be a combination of different materials. All these types of nanoparticles are highly recommended for the generation of the drug in different fields of medicine and science. Selecting nanoparticles as important and unique drug material is because of a few properties such as a much larger surface mass ratio, capability to carry and adsorb drugs, proteinase, and probes, and promotion of the reaction catalytically.[19]
ADVANTAGES OF USING NANOPARTICLES AS DRUG MATERIAL
Surface characteristics and particle size targeting the active and passive drug on parental administration are easy to manipulate. The nanoparticles provide easy higher therapeutic efficacy with fewer adverse effects, while transportation, they sustain and release the drugs at the target site, with altered drug distribution and clearance. Nanoparticles achieve the site-specific target by attracting the ligands on their surface. These particles can be administered by different routes such as intraocular, oral, nasal, and parenteral. Nanoparticles can achieve tiny sites easily because of their specific size. On the recommendation of the engineers, the researchers use these scales precisely and use these polymers based on their physical features and previously controlled biomaterials. Because of the tiny particle size, the nanoparticles easily deliver the drugs beyond the physiological barriers. Efficient delivery of the drugs is done by the nanoparticles because they are soluble in water and are capable to enhance the bioavailability of the drugs and can target the drug accurately. Nanoparticles can provide highly efficient and reduced drug toxicity. Nanoparticles can even be very helpful for the successful delivery of different biotechnological drugs to the anatomical extremities in the body such as BBB or the blood–brain barrier.
TYPES OF NANOPARTICLES
Nanoparticles are classified based on their morphology, size, chemical, and physical properties. Nanoparticles are enlisted in Table 2 based on their types.
Carbon-based nanoparticles
Carbon-based nanoparticles mainly consist of two materials fullerenes and carbon nanotubes. The carbon nanotubes are rolled sheets of graphene in a tube. Graphene is about 100 times stronger than steel. These particles are divided into two groups, namely, multi-walled carbon nanotubes and single-walled carbon nanotubes. The carbon-based nanoparticles are different based on their thermal conductive length and non-conduction through the tube whereas the fullerenes are carbon allotropes either pentagonal or hexagonal, with a hollow enclosure of 60 carbon atoms or more. The 60 –C structure reveals their similarity with football, they are known as buckminsterfullerene. These particles are the conductor of the electricity and have high electron affinity with high strength and structure.[20]
Ceramic nanoparticles
Ceramic nanoparticles are made up of carbides, oxides, phosphates, and carbonates. The ceramic-based nanoparticles possess high resistance to heat and are chemically inert. They can be used for the photodegradation of dyes, imaging, drug delivery, and photocatalysis. The ceramic-based nanoparticles can work as a good delivery agent when used and controlled according to their surface area, size, porosity, and surface volume. These are successfully used in glaucoma, bacterial infections, and cancer.[21]
Metal nanoparticles
The metal-based nanoparticles are prepared based on the metal precursors. These particles can be synthesized electrochemically, chemically, or photochemically. The chemical process of forming metal-based nanoparticles is based on the reduction of the metal ion precursors of the chemical-based reducing agents. These particles can absorb the smaller molecules with higher surface energy. These particles are used in the field of medicine for imaging and detecting the biomolecules in the body as well as the environment. They are gold, silver, alloy, and magnetic nanoparticles.
Silver-based nanoparticles
These particles are most effective and have very good antimicrobial efficiency against viral, bacterial, and eukaryotic microorganisms.[22,23] Silver is the most widely used nanoparticle used as an antimicrobial agent in sunscreen, the textile industry, and water treatment.[24,25] Successful production of the nanoparticles is reported using the plants such as Carica papaya,[26] Azadirachta indica,[27] and Capsicum annum.[28]
Gold-based nanoparticles
Gold nanoparticles are used for the identification of the interaction of proteins in immunochemical studies. Gold particles are used in the laboratory as samples in the process of DNA fingerprinting to detect the existing DNA. By the use of nanoparticles, aminoglycoside antibiotics such as gentamycin, streptomycin, and neomycin are detected. Gold nanorods are also used for the detection of cancer, stem cell cancer, and bacterial identification.[29-31]
Alloy-based nanoparticles
The mechanical properties of nanoparticles made up of alloy are different from that of bulk samples. The most often used[32-34] alloy-based nanoparticles are silver flakes because of the highest conductivity of the electric current due to the greater relative conductivity of the metallic oxides.[32] The bimetallic nanoparticles are more advantageous.[33]
Magnetic-based nanoparticles
Biocompatibility is seen in magnetic nanoparticles. They are used for guided delivery of drugs, Magnetic Resonance Imaging (MRI), gene therapy, treatment of cancer, analysis of Deoxyribonucleic Acid (DNA), sorting of stem cells, and manipulation.[34]
Semiconductor nanoparticles
Semiconductor nanoparticles can be metallic as well as non-metallic. Metals were found in groups 24, 35, or 44. Semiconductors such as Galium Phosphate (GaP), Galium Nitrite (GaN), Indium Phosphide (InP), Indium Arsenid (InAs) and Zinc oxide (ZnO), Cadmium Sulphide (CdS), Zinc Sulphide (ZnS), Cadmium Telluride (CdTe), and Cadmium Selenide (CdSe) along with germanium and silicon are used.
Polymeric nanoparticles
These are the preparation of the organic nanoparticles which depend on the shape of nanospheres and nanocapsules. These particles are active polymers which as universally dispersed active compounds which are surrounded by polymers. These are used for drug protection, imaging combined therapy, and targeting specific functions. These particles are capable to deliver highly biocompatible and biodegradable nanoparticles.
Lipid-based nanoparticles
These nanoparticles are spherical with lipophilic particles of a diameter of 10–100 nm. These are specifically used for RNA release-based treatment of cancer cells.
CONCLUSION
At the time of prescription, the clinicians and pharmacist should have complete details of newly discovered medicine as H2 blocker drugs and different nanoparticles. Interaction of different drugs, their availability, structures, and mode of interaction must be restudied thoroughly before initiating the treatment. This review provides complete information, about the generic names, scoring on the scale, and recommended doses of different H2 blockers. It also provides detailed information on the different types of available nanoparticle-based drugs. The clinicians and pharmacists must inform the patients about the toxic effects of drugs before providing them. Educational materials should be provided to the patients so that they can recognize the generic medicines. This review is an endeavor to decrease the information gap and avoid the non-prescribed distribution of such drugs or self-medication. Unaware dangerous interaction of drugs due to self-medication results in an avoidable medical emergency, which should be strictly restricted.
Ethical compliance
All procedures performed in studies involving human participants were following the ethical standards of the Institutional and National Research Committee.
Declaration of patient consent
Patients’ consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A double-blind, placebo-controlled study of the efficacy and safety of non-prescription ranitidine 75 mg in the prevention of meal-induced heartburn. Aliment Pharmacol Ther. 1999;13:467-73.
- [CrossRef] [PubMed] [Google Scholar]
- Antisecretory treatment for pediatric gastroesophageal reflux disease a systematic review. Arq Gastroenterol. 2017;54:271-80.
- [CrossRef] [PubMed] [Google Scholar]
- Management of gastroesophageal reflux disease in adults: A pharmacist's perspective. Integr Pharm Res Pract. 2018;7:41-52.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of gastroesophageal reflux disease. Pharm World Sci. 2005;27:432-5.
- [CrossRef] [Google Scholar]
- Histamine2-receptor antagonists: Standard therapy for acid-peptic diseases. N Engl J Med. 1990;323:1672-80.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetic interactions of cimetidine 1987. Clin Pharmacokinet. 1987;12:321-66.
- [CrossRef] [PubMed] [Google Scholar]
- Ranitidine drug interactions a literature review. Pharmacol Ther. 1987;32:293-325.
- [CrossRef] [Google Scholar]
- Comparative effect of famotidine and cimetidine on the pharmacokinetics of theophylline in normal volunteers. Br J Clin Pharmacol. 1987;24:669-72.
- [CrossRef] [PubMed] [Google Scholar]
- Drug interactions of H2-receptor antagonists. Scand J Gastroenterol. 2009;206:14-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J Biol Regul Homeost Agents. 2020;34:1629-32.
- [Google Scholar]
- Famotidine inhibits toll-like receptor 3-mediated inflammatory signaling in SARS-CoV-2 infection. J Biol Chem. 2021;297:100925.
- [CrossRef] [PubMed] [Google Scholar]
- Famotidine against SARS-CoV2: A hope or hype? Mayo Clin Proc. 2020;95:1797-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am J Gastroenterol. 2020;115:1617-23.
- [CrossRef] [PubMed] [Google Scholar]
- Does famotidine reduce the risk of progression to severe disease, death, and intubation for COVID-19 patients? A systemic review and meta-analysis. Dig Dis Sci. 2021;66:3929-37.
- [CrossRef] [PubMed] [Google Scholar]
- Optical sensors based on silver nanoparticles for determination of pharmaceuticals: An overview of advances in the last decade. Talanta. 2020;217:121071.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of breast cancer by analysis of sialic acid concentrations in human saliva by surface-enhanced Raman spectroscopy of silver nanoparticles. Nano Res. 2017;10:3662-70.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using cycas leaf. Int J Green Nanotech. 2010;1:110-7.
- [CrossRef] [Google Scholar]
- Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12:908-31.
- [CrossRef] [Google Scholar]
- Ceramic nanoparticles: Fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21:6165-88.
- [CrossRef] [PubMed] [Google Scholar]
- Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2:R17-71.
- [CrossRef] [PubMed] [Google Scholar]
- Toxicological hazards of inhaled nanoparticles-potential implications for drug delivery. J Nanosci Nanotechnol. 2004;4:521-31.
- [CrossRef] [PubMed] [Google Scholar]
- Protein nanoparticle: A unique system as drug delivery vehicles. Afr J Biotechnol. 2008;25:4926-34.
- [Google Scholar]
- Nanocarriers: Promising vehicle for bioactive drugs. Biol Pharm Bull. 2006;29:1790-8.
- [CrossRef] [PubMed] [Google Scholar]
- Silver Nanoparticles as a New Generation of Antimicrobials. Biotech Adv. 2009;27:813-7.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation and antibacterial activity of Fe3O4 at Ag nanoparticles. Nanotechnology. 2007;18:604-11.
- [CrossRef] [Google Scholar]
- Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv Colloid and Interface Sci. 2009;145:83-96.
- [CrossRef] [PubMed] [Google Scholar]
- Biological synthesis of triangular gold nanoprisms. Nat Mater. 2004;3:482-8.
- [CrossRef] [PubMed] [Google Scholar]
- Potential targetability of multi-walled carbon nanotube loaded with silver nanoparticles photosynthesized from Ocimum tenuiflorum (tulsi extract) in fertility diagnosis. J Drug Target. 2017;25:616-25.
- [CrossRef] [PubMed] [Google Scholar]
- Electrocatalytic properties of carbon nanotubes decorated with copper and bimetallic CuPd nanoparticles. J Phys Chem C. 2011;115:9403.
- [CrossRef] [Google Scholar]
- Biomorphic mineralization: From biology to materials. Prog Mater Sci. 2009;54:542-659.
- [CrossRef] [Google Scholar]