Translate this page into:
Impact of Vitamin D on Expression of SIRT7 and CYP24A1 in Human Breast Cancer Cells

*Corresponding author: Mahnaz Noourbakhsh, Faculty of Biological Science, University of Tehran, Tehran, Iran. m.noourbakhsh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mojarad MA, Mojarad MA, Noourbakhsh M. Impact of Vitamin D on the expression of SIRT7 and CYP24A1 in human breast cancer cells. Res Vet Sci Med 2022;2:1.
Abstract
Objectives:
Impact of vitamin D (1, 25 dihydroxy vitamin D3) (calcitriol) in the regulation of different genes has been investigated in different cancers including breast cancer (BC).
Material and Methods:
In the current study, we analyzed the expression levels of the CYP24A1 and SIRT7 genes and their relationship with patients’ clinical data in BC using polymerase chain reaction (PCR). Afterward, we tend to analyze the effect of vitamin D on the expression of these genes in cell lines (MCF7 and MDAMB231) to find the regulatory role of vitamin D in BC.
Results:
Our results showed that the CYP24A1 and SIRT7 were increased by vitamin D treatment and CYP24A1 levels were related to tumors stages (p = 0.03) and up-regulation of CYP24A1, SIRT7 had the distinguish potential for breast cancer detection based on their receiver operative characteristic (ROC) curve results (0.77, 0.84, respectively).
Conclusion:
In summary, CYP24A1, SIRT7 may be used as a possible diagnostic biomarker in BC treatment.
Keywords
Breast cancer
SIRT7
CYP24A1
Vitamin D
INTRODUCTION
Breast cancer (BC) remains primary cancer in women worldwide, both in developed and developing countries.[1,2] Despite the recent treatment approaches, including surgery, endocrine therapy, and targeted therapy, the total number of people diagnosed with cancer has nearly doubled in the past two decades.[3] Therefore, a deeper understanding of the BC pathogenesis process in molecular signaling pathways is of crucial importance.[4] Sirtuin seven (SIRT7) is one of the seven members of the sirtuin family (SIRT1–7) and belongs to Class III histone protein deacetylase (HDAC), a type of oxidized nicotinamide adenine dinucleotide (NAD +)-dependent deacetylase.[5] Sirtuins were first discovered in yeast to change replication time.[6] SIRT7 is found primarily in the nucleus with histone deacetylase activity and non-histone proteins.[7] SIRT7 has different physiological functions such as neurogenesis and cell survival, genomic stability by interacting between various proteins and is believed to have an essential role in the regulation of DNA damage repair.[8] Recent studies have shown that SIRT7 can modulate the activation of RNA polymerase I and III and influence the transcription of ribosomal RNA and Polymerase II, as well as the biosynthesis of proteins such as GABPβ1,[9] and the acetylation activity of p53.[10] Cytochrome P450 subfamilies Family 24, Part 1 (CYP24A1) is found in the inner part of the mitochondrial layer of cytochrome P450 and encodes hydroxylase 24, the protein necessary to inactivate the dynamic form of Vitamin D (1, 25-dihydroxyvitamin D3) (Calcitriol) and controls the synthesis of Vitamin D.[11,12] The main function of Vitamin D is to regulate osteosynthesis and calcium homeostasis.[13] Dietary Vitamin D converts to calcitriol which is a steroid hormone in the liver.[12] Different studies have been conducted to identify the role of Vitamin D in cancer prevention, based on its relationship with different prognostic factors such as tumor stage, degree, size, and lymph node involvement.[14,15] Recently, an inverse relationship between Vitamin D concentration in plasma and cancer incidents has been discovered.[16] Calcitriol can modify genes expression and different signaling pathways.[17] A recent discovery indicates the regulatory role of calcitriol on different genes expression and microRNAs expressions.[17] The meta-analyses study has shown that a high level of circulating 25 (OH) D can reduce BC incidence in women.[18]
Calcitriol can influence cell differentiation and proliferation by regulating the expression of many factors in tissue physiological conditions.[19] Studies have established the connection between the concentration of Vitamin D and carcinogenesis, in vivo and in vitro.[14,16,17]
CYP24A1 is an oncogene in human cancer and affects the level of expression of several signaling molecules related to carcinogenesis. Different studies have elucidated the important effect of CYP24A1 in the dysregulation of cell growth by reducing calcitriol concentrations. The loss of function mutations in CYP24A1 leads to increased levels of active Vitamin D metabolites.[19-21] Therefore, discovering how Vitamin D affects cancer initiation will help to develop efficient therapeutic strategies in BC.[22] In this work, we investigate the expression levels of CYP24A1, SIRT7, and their clinical connection in BC tissue. After Vitamin D treatment, we also examined the expression level of this gene in BC cell lines (MCF7 and MDAMB231) to understand the impact of Vitamin D on the expression of the CYP24A1 and SIRT7 genes.
MATERIAL AND METHODS
Tissue and clinical data collection
A total of 30 BC patients were included in this study between 2019 and 2020. Tumor tissues and the paired adjacent tissues were evaluated by two experienced pathologists and were quickly solidified after collection. No chemotherapy or radiotherapy before surgery was applied during diagnosis until surgery. Clinical data were collected retrospectively. Rasoul Akram Hospital approved the study, and all patients provided informed consent.
Cell lines and culture conditions
The human BC MCF-7 and MDA-MB-231, and MCF-10A cell lines were purchased from Pasteur Institute (Tehran, Iran). RPMI1640 medium (Gibco) containing 10% (vol/vol) fetal bovine serum and 1% penicillin/streptomycin (Sigma, St. Louis, MO) was used for cell culture in an incubator humidified with 5% CO2 at 37°C. Briefly, MCF7 and MDAMB231 cells (1 × 105 per well) were seeded in six-well plates and treated with 10 μM 1.25(OH)2D3 and collected 48 h after treatments.[23]
RNA isolation and polymerase chain reaction (q RT-PCR)
For RNA extraction and purification, tissues were brought out from a –70°C freezer and RNA extraction was performed using of RNeasy mini kit (Qiagen). RNA qualification and quantification were evaluated and RNA purity was graded according to the A260/A280 ratio. cDNA was synthesized from 1 μg of RNA using the Revert Aid First Strand cDNA synthesis kit according to the manufacturer’s instructions (Thermo Fisher Scientific). Next, the qRT-PCR was performed using the ABI StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster, CA) using a ×2.0 Real Q-PCR Master Mix® with SYBR Green (Amplicon, Odense, Denmark) using the following conditions: One cycle at 95°C for 15 min, 40 cycles at 95°C for 15 s, and 60°C for 60 s. Primer sets of this study the primers were designed by primer3 software are described in [Table 1]. The mRNA beta2 microglobulin (β2M) levels were used for the standardization of our reactions.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| CYP24A1 | CATTTGGCTCTTTGTTGGATTGTG | CACCATCTGAGGCGTATTATCG |
| SIRT7 | TGGAGTGTGGACACTGCTTCAG | CCGTCACAGTTCTGAGACACCA |
| β2M | TGTCTTTCAGCAAGGACTGGT | TGCTTACATGTCTCGATCCCAC |
Statistical analysis
The qRT-PCR enhancement productivity was surveyed by utilizing the LinRegPCR computer program, and for each test, we analyzed the test information as the mean ± SD, using Student’s t-test and ANOVA by utilizing GraphPad Prism 8.0 (GraphPad Program, La Jolla, CA). The expression data (ΔCt values) were examined for normality using the Kolmogorov–Smirnov test. We determined the diagnostic power of our candidate genes with receiver operating characteristic (ROC) curve analysis. Unpaired Student’s t-test was used to analyze the association between the expression levels and the clinicopathological features of the BC patients. (Statistically significant was lower than P < 0.05).
RESULTS
Detection of CYP24A1 and SIRT7, mRNA, and in tissues and cell lines
To define the role of Vitamin D (1,25-dihydroxyvitamin D3) on the expression of CYP24A1 and SIRT7, we measured CYP24A1, SIRT7, and expression levels using qRT-PCR.
CYP24A1 and SIRT7 were both measured in tissues and cell lines. CYP24A1 and SIRT7 expression was significantly upregulated in BC tissues compared with paired normal adjacent tissues (P < 0.01) [Figure 1], and in cell lines [Figure 2], compared to normal cell lines MCF-10A before and after the Vitamin D treatment. The results showed Vitamin D stimulates CYP24A1 and SIRT7 expression in BC.
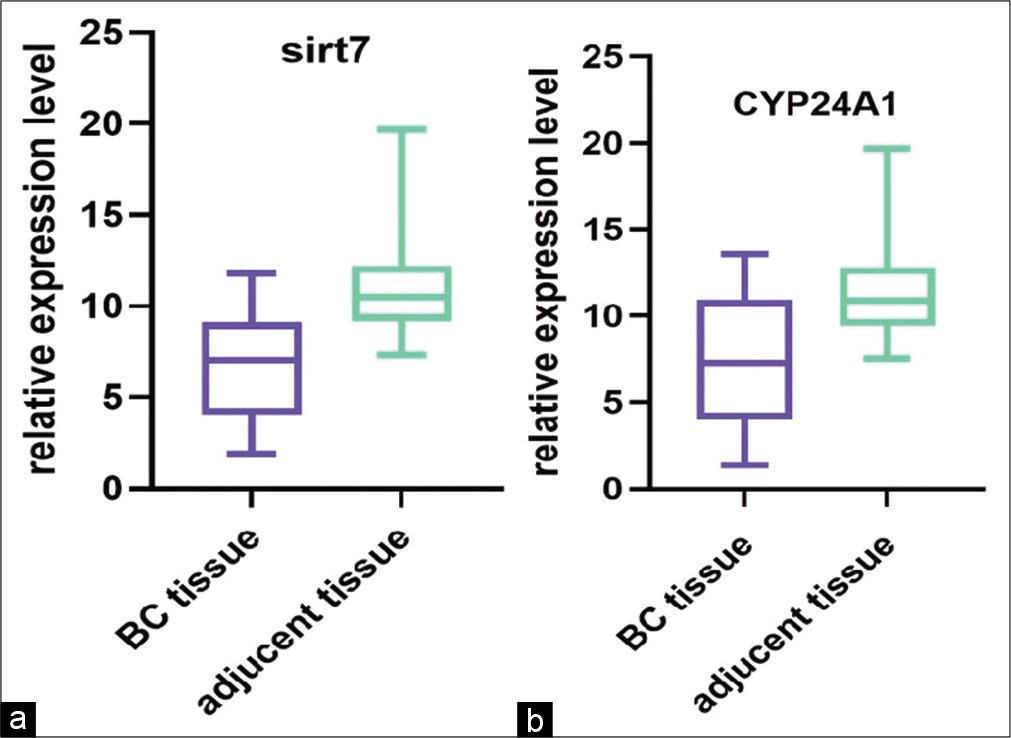
- (a and b) The relative expression level (ΔCt) of CYP24A1 and SIRT7 in breast cancer (BC) samples compared with controls. The statistics indicate that these genes were upregulated in BC samples compared with controls (P < 0.01)
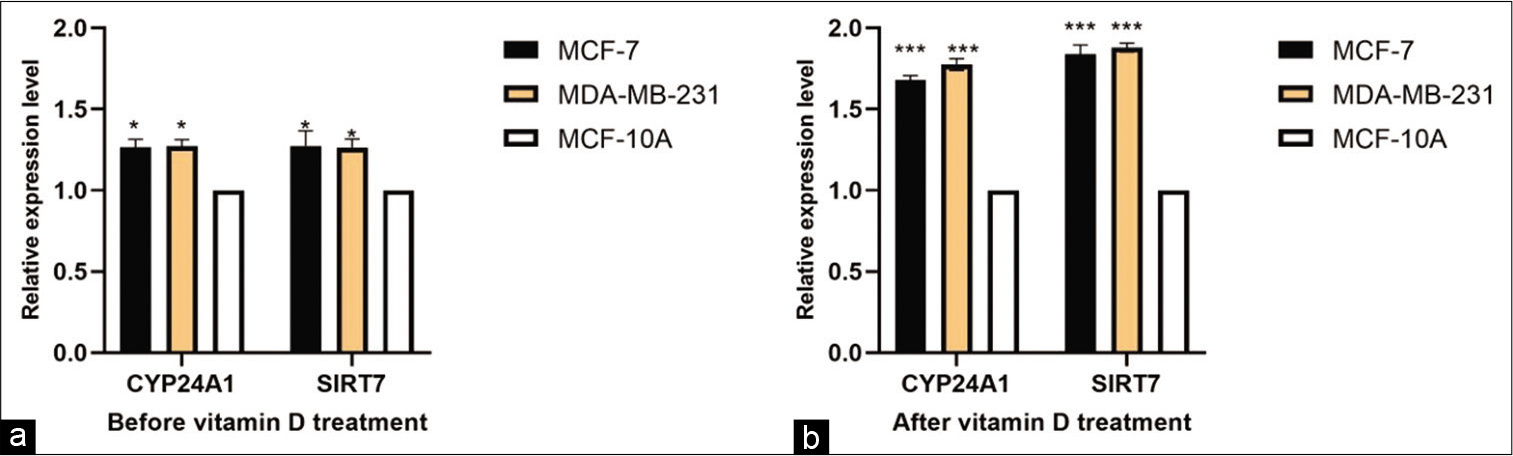
- Vitamin D (1,25-dihydroxyvitamin D3) affects the expression of CYP24A1 and SIRT7. Expression profile of CYP24A1 and SIRT7 in BC cell lines (MC7- MDA-MB-231) before Vitamin D treatment (P < 0.05) (a) and after Vitamin D treatment (P < 0.0001) (b).
Correlation between clinicopathological characteristics and the gene expression level
The correlations between the expression of CYP24A1 and SIRT7 genes and clinicopathological features in 30 BC patients are shown in [Tables 2 and 3], respectively. Our data showed that CYP24A1 was significantly correlated with tumor staging (P = 0.03). In comparison, SIRT7 has no meaningful correlation with other clinicopathological characteristics.
| Feature | No. | Low | High | P-value |
|---|---|---|---|---|
| Age | ||||
| ≤50 | 18 | 10 | 8 | 0.78 |
| >50 | 12 | 7 | 5 | |
| Tumor size (cm) | ||||
| ≤3 | 12 | 7 | 5 | |
| >3 | 18 | 9 | 9 | 0.68 |
| TNM staging | ||||
| I–II | 10 | 5 | 5 | 0.03* |
| III–IV | 20 | 10 | 10 | |
| Lymphoid node infiltrated | ||||
| Yes | 11 | 7 | 4 | |
| No | 19 | 8 | 11 | 0.21 |
| HER2+ | 11 | 6 | 5 | 0.55 |
| HER2- | 19 | 10 | 9 | |
| ER+ | 22 | 10 | 12 | 0.15 |
| ER- | 8 | 6 | 2 | |
| PR+ | 17 | 6 | 11 | 0.11 |
| PR- | 13 | 10 | 3 |
| Feature | No. | Low | High | P-value |
|---|---|---|---|---|
| Age | ||||
| ≤50 | 18 | 10 | 8 | 0.78 |
| >50 | 12 | 7 | 5 | |
| Tumor size (cm) | ||||
| ≤3 | 12 | 7 | 5 | |
| >3 | 18 | 9 | 9 | 0.68 |
| TNM staging | ||||
| I–II | 10 | 5 | 5 | 0.17 |
| III–IV | 20 | 10 | 10 | |
| Lymphoid node infiltrated | ||||
| Yes | 11 | 7 | 4 | 0.21 |
| No | 19 | 8 | 11 | |
| HER2+ | 11 | 6 | 5 | 0.55 |
| HER2- | 19 | 10 | 9 | |
| ER+ | 22 | 10 | 12 | 0. 3 |
| ER- | 8 | 6 | 2 | |
| PR+ | 17 | 6 | 11 | 0.11 |
| PR- | 13 | 10 | 3 |
The potential diagnostic values of CYP24A1 and SIRT7 in BC
We analyzed the potential diagnostic values of CYP24A1 and SIRT7 in BC. ROC curve analysis was conducted to predict the potential of CYP24A1 and SIRT7 expression as a novel diagnostic or prognostic bio markers between tumor tissues and control [Figure 3]. The area under the ROC curve for CYP24A1 and SIRT7 was 0.77 and 0.84, respectively, and the sensitivity and specificity of CYP24A1 and SIRT7 were 0.75 and 0.63 for CYP24A1; 0.83 and 0.73 for SIRT7.
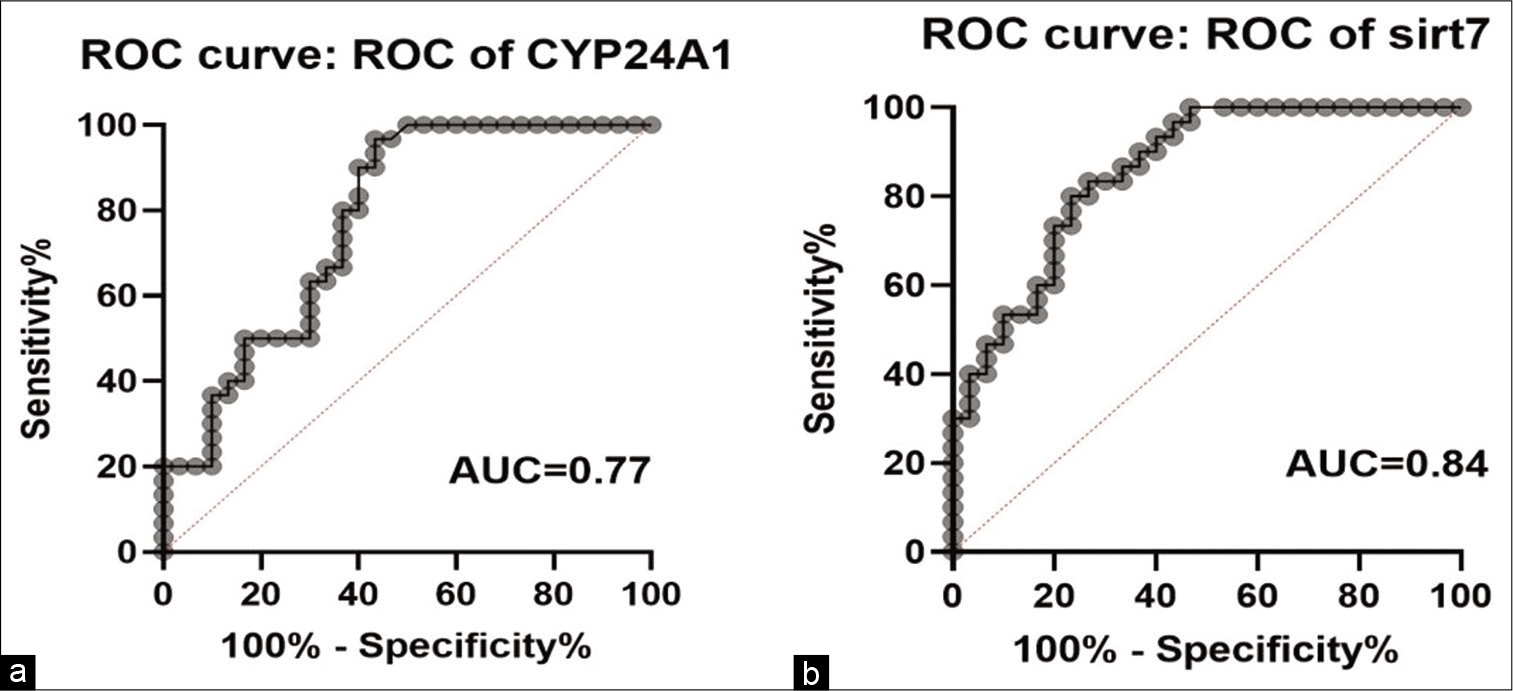
- Potential diagnostic values of CYP24A1 and SIRT7 in breast cancer. AUCs of CYP24A1 and SIRT7 were 0.77 and 0.84, respectively. With the sensitivity of 0.75 and specificity of 0.63 for CYP24A1 and 0.83 and 0.73 for SIRT7, respectively.
DISCUSSION
BC is still the most common malignancy and the first main cause of death in women, unfortunately, most patients have no specific symptoms in the early stages of BC and are diagnosed at advanced stages. Therefore, early diagnosis of the disease is essential for better treatment.[2] SIRT7 belongs to the histone deacetylase family, which can interact with different signaling pathways.[24] The role of sirtuins 7 has been reported in various pathogenesis and diseases, including cardiovascular disease, Type II diabetes, cancer, and neurodegenerative diseases.[8,9] SIRT7 expression is upregulated in metabolically active cells, and this upregulation of SIRT7 may contribute to the malignant tumor phenotype and lead to tumor genesis.[25] SIRT7 is believed to play an important role in tumor initiation, tumor progression, and the promotion of cell growth.[9] Its monogenic role has been studied in other malignancies such as human ovarian, liver, bladder, and colon cancer.[10] Mitochondrial inner membrane cytochrome enzyme CYP24A1 was nominated as an oncogene in the development of several cancers.[12] Studies have reported two Vitamin D-responsive elements (VDREs) at the upstream of the CYP24A1 gene, these VDRE sites can regulate CYP24A1 expression synergistically and play a critical role in the metabolism of Vitamin D (calcitriol) performs a different biological function, such as inducing apoptosis, modifying calcium and phosphate homeostasis, regulating hormone-dependent genes, and inhibiting proliferation.[26] Studies showed that the inhibitory role of calcitriol on cell proliferation might change to a vital role depending on the cell differentiation degree and concentration of Vitamin D. [27,28] Although the inverse association of Vitamin D and BC is studied in many meta-analyses, still there is an unexpected result about the higher concentration of 25(OH)D and BC risk.[13,29] Vitamin D also can increase the expression levels of different growth factors such as keratinocyte (KGF) and increase the proliferation ability of cancer cells.[14] Previously, we showed that the treatment of Vitamin D can modulator the expression of SIRT1 in BC cell lines (MCF-7 and MDAMB-231).[23,30] Another study has shown the role of an active form of Vitamin D in the progression of many metabolic-related diseases and showed that Vitamin D treatment can improve SIRT1 levels in serum levels of patients with Type 2 diabetes.[31] Since the significant role of calcitriol on the regulation of different genes is not fully understood, in this research, we aimed to evaluate the impact of Vitamin D on the expression of SIRT7 and CYP24A1 an enzyme related to activation of Vitamin D which both were upregulated in different tumor tissues. We also explored the correlation between these selected genes and clinic pathological characteristics in BC patients and found that the expression of CYP24A1 was significantly correlated with tumor stages (P = 0.03), and both CYP24A1 and SIRTS showed a potential to be assessed as the diagnostic biomarker in BC based on their ROC curves (0.77 and 0.84, respectively). We also determined the role of Vitamin D treatment on the regulation of CYP24A1 and SIRT7 expression in BC cell lines. Data showed Vitamin D treatments significantly induced expression of CYP24A1 and SIRT7 in both cells. In conclusion, upregulation of CYP24A1 and SIRT7 expression in tissues may play essential roles in BC progression and has the potential to be used as a diagnostic biomarker in distinguishing BC tissues from normal tissues. It may be concluded that Vitamin D treatment may modulate gene expression profile in a cell-specific manner in BC.
Data availability
The data set for this study is available from the relevant authors based on reasonable request.
Authors’ contributions
MA and MA contributed similarly to data collection, developed the paper’s idea, and revised the manuscript. The authors approved the final manuscript. MN has corresponded with this article.
Ethical approval
This study was conducted following the ethical principles of Helsinki and was approved by the TMUS Medical Ethics Committee of Tehran, Iran. All tissue samples are taken for scientific research with the written consent of the patient and their privacy will be maintained. Patients who received preoperative therapy were excluded from the study. This research was confirmed by the Tehran College of Therapeutic Sciences with the code of ethics IR.IUMS. REC.1399.9223497212.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This venture has been upheld by an investigation given by the Tehran College of Restorative Sciences (81541254).
Conflicts of interest
There are no conflicts of interest.
References
- Stimulation of sirt1-regulated foxo protein function by the ligand-bound Vitamin D receptor. Mol Cell Biol. 2010;30:4890-900.
- [CrossRef] [Google Scholar]
- Circular RNA hsa_circ_0005046 and hsa_circ_0001791 may become diagnostic biomarkers for breast cancer early detection. J Oncol. 2021;2021:2303946.
- [CrossRef] [Google Scholar]
- PDQ Adult Treatment Editorial Board, Breast Cancer Treatment (PDQ®): Patient Version United States: National Cancer Institute; 2002.
- [Google Scholar]
- Circulating non-coding RNA-biomarker potential in neoadjuvant chemotherapy of triple negative breast cancer? Int J Oncol. 2020;56:47-68.
- [CrossRef] [Google Scholar]
- Sirtuin 7 plays a role in ribosome biogenesis and protein synthesis. Mol Cell Proteomics. 2014;13:73-83.
- [CrossRef] [Google Scholar]
- SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612-8.
- [CrossRef] [Google Scholar]
- SIRT1 and estrogen signaling cooperation for breast cancer onset and progression. Front Endocrinol (Lausanne). 2018;9:552.
- [CrossRef] [Google Scholar]
- SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun. 2016;7:12235.
- [CrossRef] [Google Scholar]
- SIRT7 is a prognostic biomarker associated with immune infiltration in luminal breast cancer. Front Oncol. 2020;10:621.
- [CrossRef] [Google Scholar]
- Sirtuin 7 in cell proliferation, stress and disease: Rise of the seventh sirtuin! Cell Signal. 2015;27:673-82.
- [CrossRef] [Google Scholar]
- Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr Relat Cancer. 2017;24:339-49.
- [CrossRef] [Google Scholar]
- CYP24A1 as a potential target for cancer therapy. Anticancer Agents Med Chem. 2014;14:97-108.
- [CrossRef] [Google Scholar]
- Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010;70:1835-44.
- [CrossRef] [Google Scholar]
- Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci. 2009;46:190-209.
- [CrossRef] [Google Scholar]
- Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to Vitamin D3-based therapies. FASEB J. 2014;28:364-72.
- [CrossRef] [Google Scholar]
- Current progress in using Vitamin D and its analogs for cancer prevention and treatment. Expert Rev Anticancer Ther. 2012;12:811-37.
- [CrossRef] [Google Scholar]
- The role of Vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;145:342-57.
- [CrossRef] [Google Scholar]
- Pre-diagnostic Vitamin D concentrations and cancer risks in older individuals: An analysis of cohorts participating in the CHANCES consortium. Eur J Epidemiol. 2016;31:311-23.
- [CrossRef] [Google Scholar]
- CYP24A1- induced Vitamin D insufficiency promotes breast cancer growth. FASEB J. 2020;34:1.
- [CrossRef] [Google Scholar]
- Plasma Vitamin D levels, menopause, and risk of breast cancer: Dose-response meta-analysis of prospective studies. Medicine (Baltimore). 2013;92:123-31.
- [CrossRef] [Google Scholar]
- Impact of CYP24A1 overexpression on growth of colorectal tumor xenografts in mice fed with Vitamin D and soy. Int J Cancer. 2016;138:440-50.
- [CrossRef] [Google Scholar]
- Role of distal upstream sequence in Vitamin D-induced expression of human CYP24 gene. Biochem Biophys Res Commun. 2007;358:259-65.
- [CrossRef] [Google Scholar]
- Vitamin D has a synergistic effect on the expression levels of SIRT1 and CYP24A1 in human breast cancer. Precis Med Res. 2021;3:11.
- [CrossRef] [Google Scholar]
- Human SIRT1: A potential biomarker for tumorigenesis? Cell Biol Int. 2007;31:636-7.
- [CrossRef] [Google Scholar]
- CYP24, the enzyme that catabolizes the antiproliferative agent Vitamin D, is increased in lung cancer. Int J Cancer. 2006;119:1819-28.
- [CrossRef] [Google Scholar]
- Quantification of mRNA for the Vitamin D metabolizing enzymes CYP27B1 and CYP24 and Vitamin D receptor in kidney using real-time reverse transcriptase-polymerase chain reaction. J Mol Endocrinol. 2003;31:123-32.
- [CrossRef] [Google Scholar]
- Vitamin D3 versus gliadin: A battle to the last tight junction. Dig Dis Sci. 2018;63:1-3.
- [CrossRef] [Google Scholar]
- Vitamin D signaling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684-700.
- [CrossRef] [Google Scholar]
- Activation of raf mitogen-activated protein kinase signaling pathway by 1,25-dihydroxyvitamin D3 in normal human keratinocytes. J Invest Dermatol. 1996;106:1212-7.
- [CrossRef] [Google Scholar]
- Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 2013;6:221-31.
- [CrossRef] [Google Scholar]






