Translate this page into:
Prophylactic Anticoccidial Effects of the Methanol Extracts of Ganoderma lucidum, Vernonia amygdalina Leaves and Vitellaria paradoxa Stem Bark: An Investigation of the Cytokine and Immunoglobulin Y Responses in Eimeria tenella-infected Broiler Chickens

*Corresponding author: Paul Terkende Hambesha, Department of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria. terhambe@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hambesha PT, Orakpoghenor O, Abdu PA, Jatau ID, Aluwong T. Prophylactic Anticoccidial Effects of the Methanol Extracts of Ganoderma lucidum, Vernonia amygdalina Leaves and Vitellaria paradoxa Stem Bark: An Investigation of the Cytokine and Immunoglobulin Y Responses in Eimeria tenella-infected Broiler Chickens. Res Vet Sci Med. 2023;3:1. doi:10.25259/RVSM_5_2023
Abstract
Objectives:
This study evaluated the interleukin-6 (IL-6), interferon-gamma (INF-γ), and immunoglobulin Y (IgY) responses in Eimeria tenella-infected broiler chickens pre-administered with the methanol extracts of Ganoderma lucidum, Vernonia amygdalina leaves and Vitellaria paradoxa stem bark.
Material and Methods:
One hundred 1-day-old broiler chicks were obtained, brooded for two weeks, and then randomly divided into 10 groups (A1, B1, C1, D1, A2, B2, C2, D2, E, and F) of 10 birds each. From 14 to 20 days of age (doa), groups A1 and A2 were administered G. lucidum; B1 and B2 V. amygdalina; C1 and C2 V. paradoxa; D1 and D2 Amprolium (100 g/100 L drinking water; while no extract/medication was administered to groups E and F. At 21 doa, groups A2, B2, C2, D2, and E were challenged orally with 104 E. tenella sporulated oocysts. Blood was collected at 14, 21, and 28 doa in labeled plain tubes, and serum was harvested and assayed for IL-10, INF-γ, and IgY levels.
Results:
Results revealed no significant difference (P > 0.05) in the serum IL-10, INF-γ, and IgY levels in all groups of chickens at 14 and 21 doa. At 28 doa, serum IL-10 level was significantly (P < 0.05) higher in B2 and C2 compared to A2, D2, and E. The levels of INF-γ and IgY were not significantly (P > 0.05) higher in B2 and C2 than in A2, D2, and E. The prophylactic administration of G. lucidum, V. amygdalina leaf, and V. paradoxa stem bark methanolic extracts altered changes in the serum IL-10, INF-γ, and IgY levels induced by E. tenella challenge in the broiler chickens.
Conclusion:
The mechanisms involved in these cytokines and IgY responses as a result of the prophylactic administration of these extracts require further investigation.
Keywords
Prophylactic
Methanol extracts
Eimeria tenella
Cytokine
Immunoglobulin Y
INTRODUCTION
Coccidiosis constitutes one of the major parasitic diseases of broiler chickens, resulting in poor performance and productivity loss.[1,2] It is caused by single-celled protozoan parasites of the genus Eimeria, which are commonly referred to as Coccidia. Birds are usually infected following ingestion of sporulated Eimeria oocysts.[3] Two types of coccidiosis, clinical and subclinical, exist in poultry. In clinical coccidiosis, the affected birds show typical symptoms of the disease, such as bloody droppings and increased mortality. On the other hand, in subclinical coccidiosis, affected birds do not show visible symptoms of the disease, although evidence of the parasite and gross lesions are found following examination.[3] The control of coccidiosis in poultry has been through the use of anticoccidial drugs, vaccination, and strict management practices.[4]
The use of anticoccidial drugs has been adopted globally for the control of coccidiosis in the poultry industry. Although this adoption has seen great acceptance and success, there is continuous pressure on the poultry industry to decrease the use of antimicrobials, including anticoccidial drugs.[5] This is because there is increasing development of resistance or reduced sensitivity of Eimeria to chemotherapeutic agents due to long-term exposure.[6,7] In addition, the presence of anticoccidial residues in chicken meat[7,8] has necessitated the need to adopt alternative methods of coccidiosis control.
These alternative methods of coccidiosis control involve the use of components of natural materials such as Ganoderma lucidum, Vernonia amygdalina leaves, and Vitellaria paradoxa. Studies involving these materials against coccidiosis were based on therapeutic administration[9,10] with scanty information on their prophylactic anticoccidial administration. The pathophysiology of coccidiosis in poultry involves the activation of several aspects of the host immune system, including cytokines and immunoglobulin responses.[11,12] Thus, in this study, the interleukin-6 (IL-6), interferon-gamma (INF-γ) and immunoglobulin Y (IgY) responses in Eimeria tenella-infected broiler chickens pre-administered with the methanol extracts of G. lucidum, V. amygdalina leaves, and V. paradoxa stem bark were evaluated.
MATERIAL AND METHODS
Ethical approval
The Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC) granted the approval for this study (ABUCAUC/2022/056).
Location of the experiment
The poultry research pens of the Veterinary Teaching Hospital, Ahmadu Bello University (ABU), Zaria, Nigeria, were used for the study.
Collection and identification of pharmaceutical materials
Professor P. A. Abdu (Department of Veterinary Medicine, ABU, Zaria, Nigeria) provided the G. lucidum and V. paradoxa stem bark; at the same time the leaves of V. amygdalina were collected from a private vegetable garden in Makurdi, Benue State, Nigeria. These materials were identified in the Department of Botany, ABU, Zaria.
Processing, preparation, and phytochemical analyses of extracts
Methanol extracts of the materials were prepared[13] after processing[14] and the phytochemical constituents in each extract were identified.[15]
Source of E. tenella oocysts
A previously isolated and characterized E. tenella[16] was provided by the Department of Veterinary Parasitology and Entomology Laboratory, ABU, Zaria, Nigeria.
Experimental chickens
One hundred 1-day-old Arbor acre broiler chickens were obtained from a commercial hatchery and reared in pens containing ten wire-floored battery cages, each measuring 1,000 mm × 1,000 mm × 800 mm and raised to a height of 100 mm from the floor of the pen. The cages were cleaned, washed, disinfected, and with adequate rodent and insect control. The birds were fed with commercial broiler starter feed, and water was provided ad libitum without drugs or supplements. Before the commencement of the experiment, the chicks were brooded together for two weeks, screened, and confirmed negative for E. tenella oocyst.
Prophylactic administration of extracts to birds
The birds were administered the methanol extracts from 14 to 20 days of age (doa). G. lucidum and V. paradoxa extracts were administered at 250 mg/kg body weight,[9] while V. amygdalina extract was administered at 2,000 mg/kg body weight[10] orally for seven consecutive days. Furthermore, 100 g of amprolium hydrochloride (Amprolium 250 wsp; KEPRO B. V. – Maagdenburgstraat 17 – 7421 ZA Deventer – Holland) was administered at 100 g/100 L of drinking water for seven consecutive days.
Challenge of birds with E. tenella
Birds were orally challenged at 21 doa with 104 sporulated oocysts of E. tenella.
Experimental design
At 14 doa, the broiler chickens were randomly divided into 10 groups (A1, B1, C1, D1, A2, B2, C2, D2, E, and F) of 10 birds each. Groups A1 and A2 were administered G. lucidum extract; B1 and B2 V. amygdalina; C1 and C2 V. paradoxa. Group D1 and D2 were administered with amprolium. At 21 doa, groups A2, B2, C2, D2, and E (positive control) were challenged orally with 104 E. tenella sporulated oocysts. Group F (negative control) was neither treated nor challenged. Clinical signs were monitored. Blood was collected at 14, 21, and 28 doa into a labeled plain tube, serum was harvested, and assayed for IL-10, INF-γ, and IgY levels.
Determination of serum IL-10, INF-γ, and IgY levels
The levels of IL-10, INF-γ, and IgY were determined using IL-10, INF-γ, and IgY enzyme-linked immunosorbent assay kits (Pioway), respectively, by following the manufacturer’s instructions.
Data analyses
Data were presented as means and standard errors of means (±standard error of the mean [SEM]) on charts. The data were subjected to one-way analysis of variance Tukey’s post hoc test. Statistical Package for Social Sciences 13.0, Chicago, IL was used for the analyses. Values of P ≤ 0.05 were considered statistically significant.
RESULTS
Phytochemical constituents of extracts and clinical signs observed
The constituents identified in V. amygdalina leaves and V. paradoxa stem bark extracts were tannins, flavonoids, cardiac glycosides, alkaloids, anthraquinones, carbohydrates, saponins, steroids, and triterpenes. In G. lucidum extract, all the above constituents but alkaloids and tannins were identified.
The clinical signs observed in A2, B2, C2, D2, and E were dullness, somnolence, ruffled feathers, anorexia, and bloody and mucoid diarrhea.
IL-10 level
There was no significant (P > 0.05) difference in the serum IL-10 level in all groups at 14 and 21 doa [Figure 1]. At 28 doa, the serum IL-10 level was significantly (P < 0.05) higher in challenged (A2, B2, C2, D2, and E) than in unchallenged (A1, B1, C1, D1, and F) groups. In the challenged groups, the serum IL-10 level was significantly (P < 0.05) higher in C2 (42.83 ± 1.59 pg/mL) and B2 (40.18 ± 2.56 pg/mL) than in A2 (36.64 ± 0.98 pg/mL), D2 (36.45 ± 0.90 pg/mL) and E (35.79 ± 0.70 pg/mL) [Figure 1].
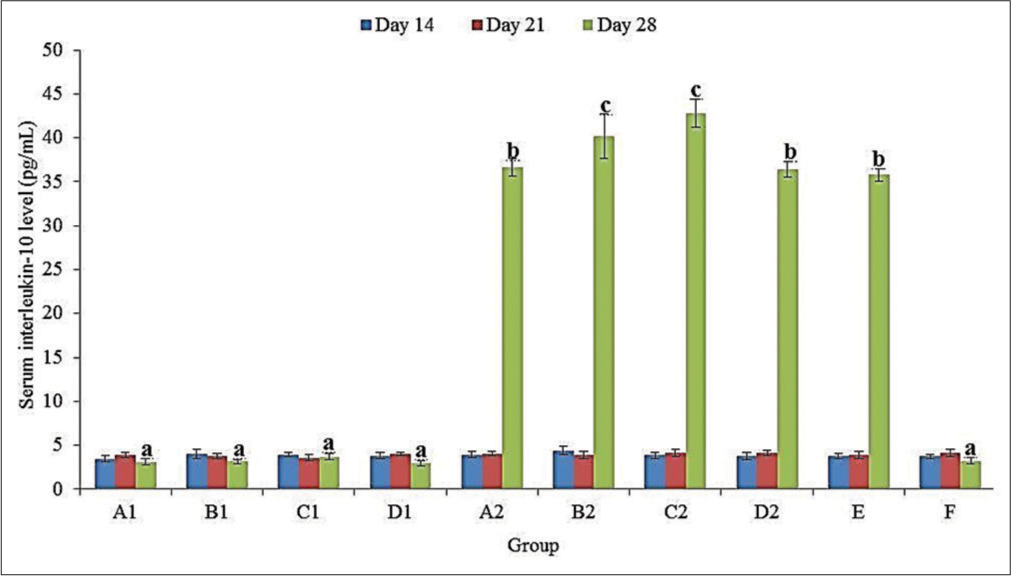
- Serum interleukin 10 levels of broiler chickens administered methanol extracts of Ganoderma lucidum, Vernonia amygdalina leaves and Vitellaria paradoxa stem bark from 14 to 20 days of age (doa), and challenged with Eimeria tenella oocysts at 21 doa. a, b, c differ significantly at P < 0.05.
INF-γ level
No significant (P > 0.05) difference in the mean serum INF-γ levels of all groups at 14 and 21 doa [Figure 2]. At 28 doa, there was a significantly (P < 0.05) higher mean (± SEM) serum INF-γ level in the challenged compared to unchallenged groups. Among the challenged groups, serum INF-γ level was non-significantly (P > 0.05) higher in groups B2 (27.08 ± 0.09 pg/mL) and C2 (26.95 ± 0.16 pg/mL) compared to A2 (26.27 ± 0.37 pg/mL), D2 (24.73 ± 1.11 pg/mL) and E (24.70 ± 1.24 pg/mL) [Figure 2].
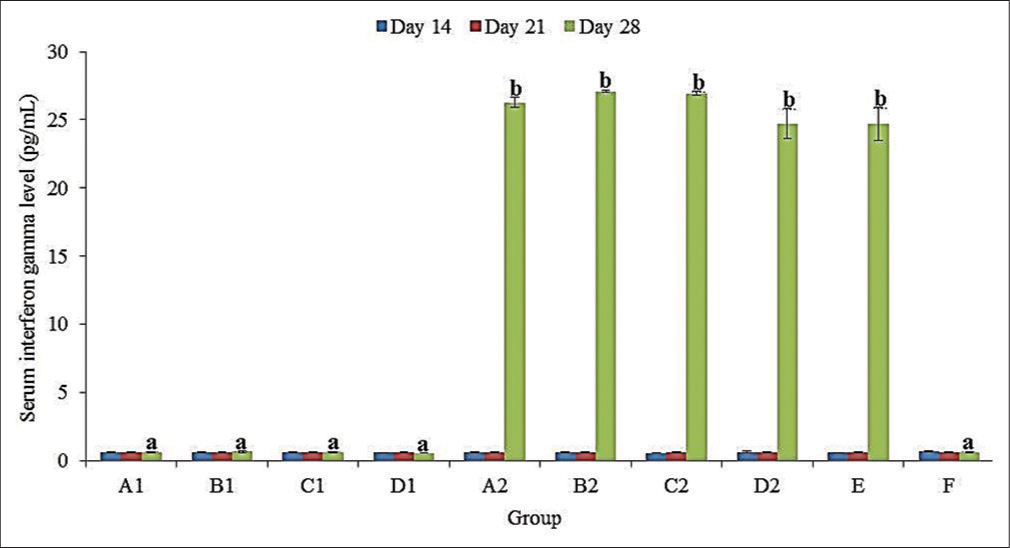
- Serum interferon-gamma levels of broiler chickens administered methanol extracts of Ganoderma lucidum, Vernonia amygdalina leaves, and Vitellaria paradoxa stem bark from 14 to 20 days of age (doa), and challenged with Eimeria tenella oocysts at 21 doa.
IgY level
There was no significant (P > 0.05) difference in the serum IgY levels of all groups at 14 and 21 doa [Figure 3]. The serum IgY level was significantly (P < 0.05) higher at 28 doa in groups A2, B2, C2, D2, and E compared to those of A1, B1, C1, D1, and F. This was non-significantly (P > 0.05) higher in groups B2 (23.77 ± 3.07 µg/mL) and C2 (24.36 ± 1.88 µg/mL) than in A2 (20.54 ± 2.24 µg/mL), D2 (20.11 ± 2.18 µg/mL) and E (18.49 ± 1.56 µg/mL) at 28 doa [Figure 3].
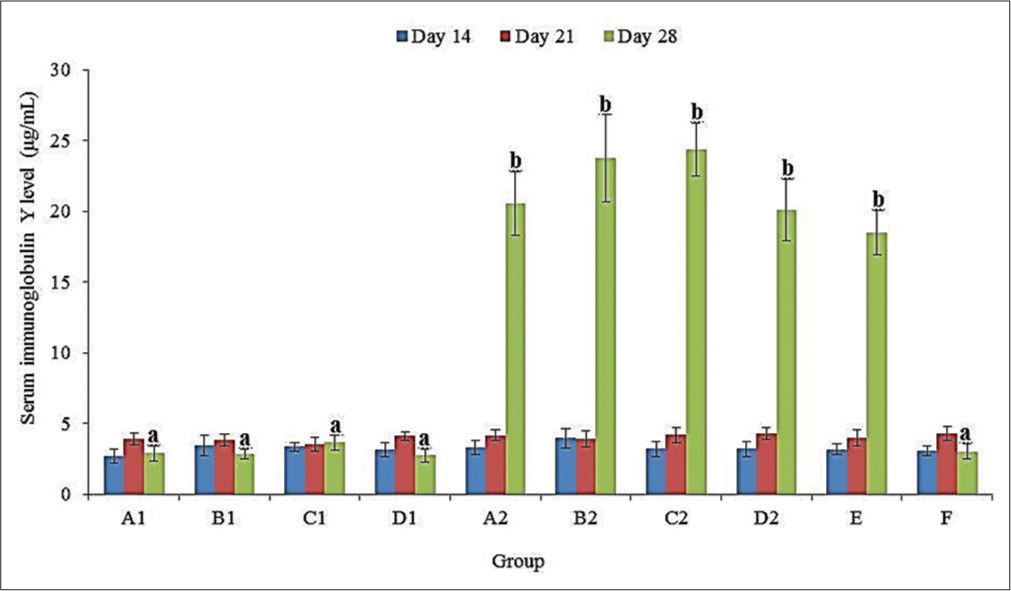
- Serum immunoglobulin Y levels of broiler chickens administered methanol extracts of Ganoderma lucidum, Vernonia amygdalina leaves, and Vitellaria paradoxa stem bark from 14 to 20 days of age (doa), and challenged with Eimeria tenella oocysts at 21 doa.
DISCUSSION
The clinical signs of coccidiosis in challenged groups (A2, B2, C2, D2, and E) of broiler chickens in this study were dullness, somnolence, ruffled feathers, decreased feed intake, bloody diarrhea, and mucoid diarrhea. These findings are consistent with the reports of other studies.[17,18] This confirmed that the characterized E. tenella isolate[16] used in this study was pathogenic to the broiler chickens.
As part of protective immunity against avian coccidiosis, T-cells were reported to produce numerous cytokines, including IL-10 and IFN-γ.[19] Following an investigation on the kinetics of host immune response to coccidiosis, the RNA-sequencing results suggested that early activation of IFN-γ, IL-10, and immune-related genes might be the key to resistance to coccidiosis.[20] IL-10 is a cytokine with potent anti-inflammatory properties that plays a central role in limiting the host immune response to pathogens, thereby preventing damage to the host and maintaining normal tissue homeostasis.[21] It plays a down regulatory role so as to reduce inflammation and host pathology during the disease process.[22] IL -10 controls the host immune response to limit off-target host cell damage during inflammation by inhibiting pro-inflammatory cytokines such as IL-12 and IFN-γ, preventing major histocompatibility II expression and suppressing nitric oxide species production.[22] In the present study, there was a significant increase in IL-10 levels in challenged groups at 28 doa, but the level was higher in groups C2 and B2 compared to A2, D2, and E. This increase in IL-10 levels in the challenged groups might be due to the E. tenella challenge, as Eimeria infection was reported to cause an increase in host caecal and systemic IL-10 levels.[23,24] The increased IL-10 level in pre-treated groups might be due to the ability of the extracts’ constituents to upregulate IL-10 expression directly and/or indirectly via interference with the cascade of events that led to IL-10 production.
Among the cytokines involved in coccidiosis, IFN-γ is suggested to be a representative immunomodulator due to its direct inhibitory effect on the intracellular development of Eimeria.[25] In this study, there was a significant (P < 0.05) increase in IFN-γ level in challenged broiler chickens and this increase was more in groups A2, B2, and C2 than in D2 and E. A study detected considerable expression of IFN-γ in the spleen and caecal tonsils but decreased IFN-γ in the duodenum of inbred chicken following Eimeria acervulina infection.[26] In another study, upregulated expression of IFN-γ was detected in the spleens, caecal tonsils, and in intraepithelial lymphocytes post-primary and secondary E. tenella infections.[27] It has been documented that IFN-γ, specifically produced by Eimeria species, stimulated peripheral blood lymphocytes in chickens with coccidiosis.[28] Hence, the increased IFN-γ level in challenged groups in this study was expected. However, the higher level in the pre-treated groups might be due to upregulated expression and/or production of IFN-γ induced by the constituents of the extracts following their prophylactic administration.
The production of parasite-specific antibodies in circulation and across mucosal surfaces of birds in response to coccidial infection was documented, but the role of humoral immunity against coccidiosis has not been elucidated.[29,30] Studies have demonstrated that antibodies play an important role in immunity against coccidiosis.[31,32] In the present study, serum IgY was significantly higher in challenged groups. This finding is consistent with the work of Davou et al.[33] who reported higher serum IgY titer in broilers chickens infected with sporulated oocyst and merozoites of E. tenella. The IgY level showed no significant difference in the challenged groups but was higher in groups A2, B2, and C2 than in D2 and E in the present study. This might be due to the immunomodulating effects of the constituents in the extracts following their prophylactic administration.
CONCLUSION
The prophylactic administration of G. lucidum, V. amygdalina leaf, and V. paradoxa stem bark methanol extracts altered changes in the serum IL-10, INF-γ, and IgY levels induced by E. tenella challenge in the broiler chickens. The mechanisms involved in these cytokines and IgY responses as a result of the prophylactic administration of these extracts require further investigation.
Ethical approval
The Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC) granted the approval for this study (ABUCAUC/2022/056).
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflict of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Prevalence and Aetiology of Coccidiosis in Broiler Chickens in Bejaia Province, Algeria. Onderstepoort J Vet Res. 2018;85:e1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Quantifying the Effect of Coccidiosis on Broiler Performance and Infection Outcomes in the Presence and Absence of Control Methods. Poult Sci. 2022;101:101746.
- [CrossRef] [PubMed] [Google Scholar]
- Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front Vet Sci. 2021;8:787653.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and Control of Chicken Coccidiosis: A Recent Update. J Parasit Dis. 2018;42:483-93.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of an Attenuated Anticoccidial Vaccine on the Intestinal Ecosystem and on the Pathogenesis of Experimental Necrotic Enteritis in Broiler Chickens. Avian Pathol. 2013;42:163-70.
- [CrossRef] [PubMed] [Google Scholar]
- A Selective Review of Advances in Coccidiosis Research. Adv Parasitol. 2013;83:93-171.
- [CrossRef] [PubMed] [Google Scholar]
- Anticoccidial Drugs of the Livestock Industry. Parasitol Res. 2019;118:2009-26.
- [CrossRef] [PubMed] [Google Scholar]
- An Update on Approaches to Controlling Coccidia in Poultry using Botanical Extracts. Br Poult Sci. 2013;54:713-27.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the Effects of Methanol Extracts of Vitellaria paradoxa Stem Bark and Ganoderma lucidum in the Treatment of Broilers Experimentally Infected with Eimeria tenella oocysts In: Msc Dissertation. Nigeria: Ahmadu Bello University Zaria; 2019. p. :136.
- [Google Scholar]
- Phytochemical Profile of the Methanolic Leaf Extract of Vernonia amygdalina Del, and in vitro Anticoccidial Effect of its Fractions against Eimeria tenella. Int J Innov Res Dev. 2022;11:67-72.
- [Google Scholar]
- Coccidiosis: Recent Advancements in the Immunobiology of Eimeria Species, Preventive Measures, and the Importance of Vaccination as a Control Tool against these Apicomplexan Parasites. Vet Med (Auckl). 2014;5:23-34.
- [CrossRef] [PubMed] [Google Scholar]
- Research Note: Expression of T Cell-related Cytokines in Chicken Cecal and Spleen Tissues Following Eimeria tenella Infection in vivo. Poult Sci. 2021;100:101161.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-protozoal Efficacy of Medicinal Herb Extracts against Toxoplasma gondii and Neospora caninum. Vet Parasitol. 2003;116:7-14.
- [CrossRef] [PubMed] [Google Scholar]
- Proximate Composition, Phytochemical and Elemental Analysis of Some Organic Solvent, Extract of the Wild Mushroom, Ganoderma lucidum. J Nat Sci Res. 2012;2:24-35.
- [Google Scholar]
- Textbook of Pharmacognosy (16th ed). United States: Saunders Ltd; 2009. p. :135-47.
- [Google Scholar]
- Three Operational Taxonomic Units of Eimeria are Common in Nigerian Chickens and may Undermine Effective Molecular Diagnosis of Coccidiosis. BMC Vet Res. 2016;12:86.
- [CrossRef] [PubMed] [Google Scholar]
- Anticoccidial Activity of Aqueous Extract of a Wild Mushroom (Ganoderma applanatum) during Experimentally Induced Coccidial Infection in Broiler Chicken. J Parasit Dis. 2016;40:408-14.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative Effect of Dietary Supplements on the Performance and Severity of Experimental Eimeria tenella Infection in Broiler Chickens. Trop Anim Health Prod. 2022;54:191.
- [CrossRef] [PubMed] [Google Scholar]
- Involvement of T Cell Immunity in Avian Coccidiosis. Front Immunol. 2019;10:2732.
- [CrossRef] [PubMed] [Google Scholar]
- Kinetics of the Cellular and Transcriptomic Response to Eimeria Maxima in Relatively Resistant and Susceptible Chicken Lines. Front Immunol. 2021;12:653085.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit Rev Immunol. 2012;32:23-63.
- [CrossRef] [PubMed] [Google Scholar]
- Investigating the Role of Interleukin 10 on Eimeria Intestinal Pathogenesis in Broiler Chickens. Vet Immunol Immunopathol. 2019;218:109934.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of Dietary Amino Acid Reductions and Eimeria acervulina Infection on Growth Performance and Intestinal Cytokine Responses of Broilers Fed Low Crude Protein Diets. Poult Sci. 2016;95:2602-14.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-10 Neutralizing Antibody for Detection of Intestinal Luminal Levels and as a Dietary Additive in Eimeria Challenged Broiler Chicks. Poult Sci. 2016;95:430-8.
- [CrossRef] [PubMed] [Google Scholar]
- Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines (Basel). 2022;10:215.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in Local IFN-gamma and TGF-beta4 mRNA Expression and Intraepithelial Lymphocytes Following Eimeria acervulina Infection. Vet Immunol Immunopathol. 1999;71:263-75.
- [CrossRef] [PubMed] [Google Scholar]
- Depression is an Inflammatory Disease, but Cell-mediated Immune Activation is the Key Component of Depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664-75.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral Blood Lymphocytes from Eimeria tenella Infected Chickens Produce Gamma-Interferon after Stimulation in vitro. Parasite Immunol. 1997;19:127-35.
- [CrossRef] [PubMed] [Google Scholar]
- Avian Gut-associated Lymphoid Tissues and Intestinal Immune Responses to Eimeria parasites. Clin Microbiol Rev. 1996;9:349-60.
- [CrossRef] [PubMed] [Google Scholar]
- Intestinal Immune Responses to Coccidiosis. Dev Comp Immunol. 2000;24:303-24.
- [CrossRef] [PubMed] [Google Scholar]
- Characterisation of the Antigenic and Immunogenic Properties of Bacterially Expressed, Sexual Stage Antigens of the Coccidian Parasite, Eimeria maxima. Vaccine. 2004;22:4316-25.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody Response against Endogenous Stages of an Attenuated Strain of Eimeria tenella. Vet Parasitol. 2008;154:193-204.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody Response in Broiler Chickens Infected with Different Developmental Stages of Eimeria tenella. SOJ Immunol. 2018;6:1-4.
- [CrossRef] [Google Scholar]







