Translate this page into:
Multidrug-Resistant Corynebacterium spp., Escherichia coli, and Klebsiella spp. Isolated from the Lungs of a Six-Year-Old Arewa Stallion

*Corresponding author: Talatu Patience Markus, Microbiology Specialty, Pathology Faculty, College of Veterinary Surgeons of Nigeria, Zaria Study Centre, Zaria, Kaduna, Nigeria. talatupmarkus@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Markus TP, Orakpoghenor O, Mamman PH. Multidrug-Resistant Corynebacterium spp., Escherichia coli, and Klebsiella spp. Isolated from the Lungs of a Six Year-Old Arewa Stallion. Res Vet Sci Med. 2025;15:1. doi: 10.25259/RVSM_15_2024
Abstract
This manuscript presents a case of multidrug-resistant (MDR) bacterial infections in the respiratory system of a6-year-old Arewa stallion, thus highlighting the importance of diagnostic microbiology. History revealed that the stallion exhibited clinical signs, including high fever, hemoglobinuria, and edema of the head and neck, and this was followed by death despite medical intervention. Postmortem was pneumonia; hence lung sample was sent for microbiological analyses. The diagnostic microbiological techniques utilized were culture on blood MacConkey and Eosin Methylene Blue agars, and biochemical tests were used to confirm the bacterial identities, followed by antibiotic susceptibility testing. The outcomes revealed the isolation of Corynebacterium spp., Escherichia coli, and Klebsiella spp. Antibiotic susceptibility testing revealed extensive resistance, with Corynebacterium spp. resistant to Septrin, Chloramphenicol, Sparfloxacin, Ciprofloxacin, and more. E. coli and Klebsiella spp. were resistant to Ciprofloxacin, Gentamicin, Nitrofurantoin, Ofloxacin, Cefuroxime, Ceftazidime, Augmentin, and Cotrimoxazole. These findings emphasize the diagnostic challenges caused by MDR pathogens in equine respiratory diseases.
Keywords
Antibiotic susceptibility testing
Cultural isolation
Diagnostic microbiology
Equine respiratory diseases
Multidrug resistance
INTRODUCTION
Diagnostic microbiology plays a crucial role in veterinary medicine by enabling the identification of pathogens responsible for infectious diseases, thus guiding appropriate treatment strategies.[1] In equine practice, respiratory infections are a significant concern due to their impact on animal health and performance.[2] The emergence of multidrug-resistant (MDR) bacteria poses a severe challenge to treatment efficacy, thus making timely and accurate microbial diagnosis even more critical.[3] This report highlights the complexities of managing a case of MDR bacterial infection in a 6-year-old Arewa stallion, emphasizing the importance of diagnostic microbiology in devising effective therapeutic interventions.
This case report provides critical insights into the diagnostic challenges posed by MDR bacterial infections in equine respiratory diseases. The findings align with current trends in veterinary microbiology, thus highlighting the need for comprehensive diagnostic protocols, including necropsy, culture, biochemical testing, and antibiotic susceptibility testing. By presenting this case, we aim to shed light on the evolving scope of antibiotic resistance in equine respiratory diseases and the pivotal role of diagnostic microbiology in addressing these challenges, to ultimately improve animal health outcomes.
CASE REPORT
A lung sample was obtained from the carcass of a 6-year-old Arewa stallion presented to the Necropsy Unit, Veterinary Teaching Hospital, and submitted to the Bacteriology Laboratory, Department of Veterinary Microbiology, Ahmadu Bello University Zaria, Nigeria. History revealed that the nail was removed from the left forelimb, and tetanus toxoid was administered 3 weeks before the presentation of the stallion to the Large Animal Clinic. The clinical signs observed were hemoglobinuria, sweating, high temperature (41°C), congested ocular mucous membrane, dilation of the nostrils, restlessness, recumbency, and generalized edema around the head and neck. The medications administered were imidocarb dipropionate, benzyl penicillin, intravenous dextrose saline, xylazine, diclofenac, and multivitamin, and the feed were hay and bran (wheat offal).
The stallion died 2 days of after being sick and recumbent, and the carcass was transported to the Necropsy unit for postmortem examination. The gross findings were a slightly enlarged heart with a chicken fat clot in the right ventricle; congested, hemorrhagic and hepatized lungs; hemorrhagic trachea with frothy exudates; enlarged and congested liver; enlarged spleen; severe hemorrhagic gastroenteritis with helminths in the colon; and congested kidneys.
Microbiological examinations
Culture and isolation
The surface of the lung sample was seared with a sterilized spatula and a loop was used to culture on blood and MacConkey agars and incubated at 37°C for 24 h and later observed for colonies. Presumptive colonies of Echerichia coli were then sub-cultured onto Eosin Methylene Blue (EMB) agar.
Biochemical test
The isolates presumptive of E. coli and Klebsiella spp. was inoculated into Triple Sugar Iron, Indole, Urea, Citrate, Methyl Red, Voges Proskauer and motility and incubated for 24 h at 37°C.
Catalase test
This was performed using a sterilized loop to pick from the colony on blood agar and placing on a clean glass slide. This was followed by addition of a drop of hydrogen perioxide on the colony and observation for bubble formation which is indicative of a positive reaction.
Antibiotic susceptibility test
Disc diffusion method was used for antibiotic sensitivity testing of the isolates. Mueller–Hinton agar was used, and the gram positive was smeared with a cotton swab onto the Mueller–Hinton agar. Then, using a sterilized forcep, a Gram-positive antibiotic sensitivity disc was picked and placed onto the media and incubated for 24 h at 37°C. The same procedure was repeated for the Gram-negative isolates.
Outcome of culture and isolation
Three organisms were isolated from the lungs of the horse. The first isolate appeared as small, grayish, non-hemolytic colonies on blood agar and did not grow on MacConkey. The isolate was gram-positive rods with a Chinese letter-like appearance [Figure 1], presumptive for Corynebacterium spp.
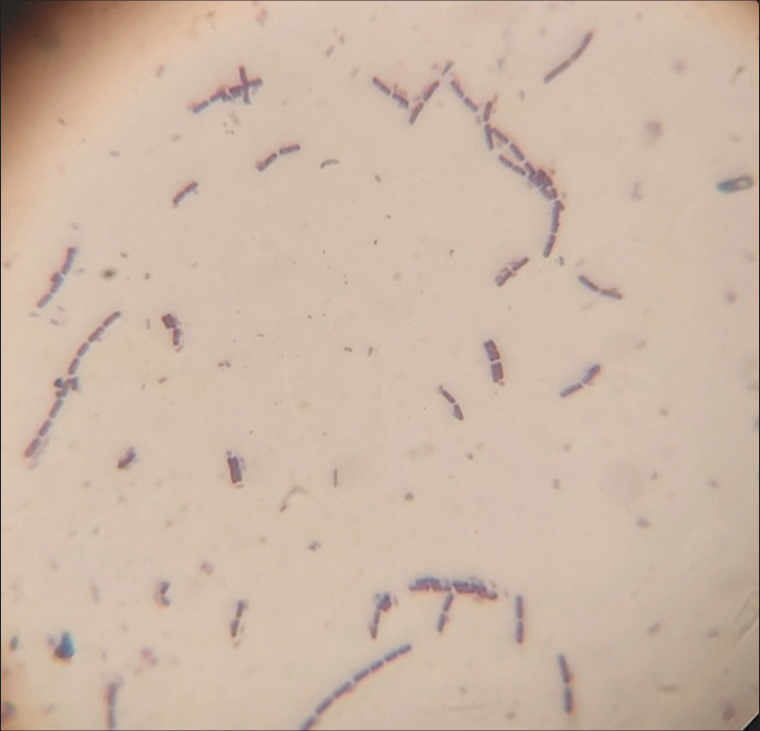
- Gram-positive rods with the shape of Chinese letters, presumptive for Corynebacterium spp (Grams stain, X100 Magnification).
The second isolate appeared as medium-sized, smooth, raised, whitish non-hemolytic colonies on blood agar, which was pinkish on MacConkey (lactose fermenter), produced a greenish metallic sheen on EMB [Figure 2a], gram-negative rods [Figure 2b], suggestive of E. coli. The third isolate appeared as gray, small, mucoid, non-hemolytic colonies on blood agar, pink, mucoid colonies on MacConkey [Figure 3a] and Gram-negative short rods [Figure 3b] presumptive for Klebsiella spp.
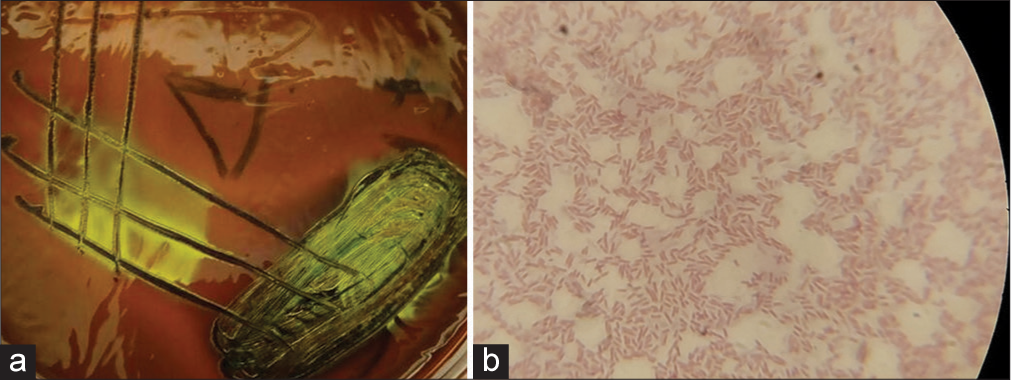
- (a) Colonies with greenish metallic sheen on eosin methylene blue agar; and (b) Gram-negative rods, presumptive for Escherichia coli (Grams stain, X100 Magnification).
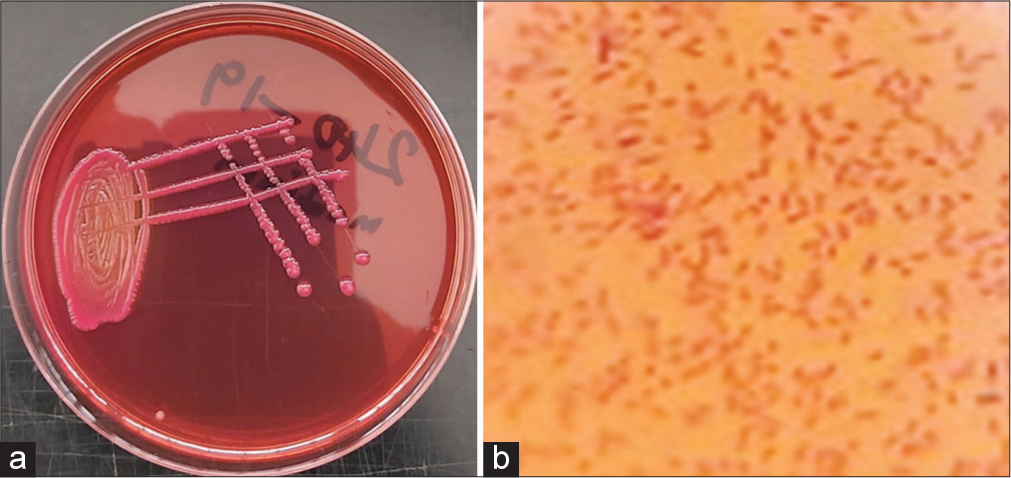
- (a) Small size, pink, mucoid colonies on MacConkey agar; and (b) Gram-negative short rods, presumptive for Klebsiella spp (Grams stain, X100 Magnification).
Biochemical test results
The biochemical test identified the second isolate as E. coli and the third as Klebsiella spp. [Table 1 and Figure 4].
| S/N | Biochemical test | Isolate II | Isolate III |
|---|---|---|---|
| 1 | TSI | Acid/acid+gas | Acid/acid+gas |
| 2 | Indole | Positive | Negative |
| 3 | Citrate | Negative | Positive |
| 4 | Urea | Negative | Positive |
| 5 | Methyl red | Positive | Negative |
| 6 | Voges Proskauer | Negative | Positive |
| 7 | Motility | Motile | Non-motile |
| Escherichia coli | Klebsiella spp. |
TSI: Triple sugar iron

- (a) Biochemical test outcome of Escherichia coli (b) Biochemical test outcome of Klebsiella spp. From L-R (a & b): Triple Sugar Iron, Indole, Urease, Citrate, Methyl Red, Voges Proskauer and motility.
Catalase test reaction
The presumptive isolate of Corynebacterium spp. was catalase positive.
Antibiotic susceptibility test
Corynebacterium spp. was resistant to all the antibiotics used (Septrin, Chloramphenicol, Sparfloxacin, Ciprofloxacin, Amoxicillin, Augmentin, Gentamicin, Pefloxacin, Tarivid, Streptomycin). E. coli and Klebsiella spp. were also resistant to all the antibiotics used (Ciprofloxacin, Gentamicin, Nitrofurantoin, Ofloxacin, Cefuroxime, Ceftazidime, Augmentin, Co-trimoxazole).
DISCUSSION
The isolation of Corynebacterium spp., E. coli, and Klebsiella spp. from the lungs of the stallion highlights the complex interplay of bacterial pathogens in severe equine respiratory infections.[4] The case presented a rapid progression of clinical symptoms, including systemic inflammation (generalized edema), severe respiratory distress, and multisystemic involvement, ultimately leading to mortality within 2 days of onset. The observed history of tetanus toxoid administration following the removal of a nail from the forelimb suggests that the animal was already immunologically challenged, potentially predisposing it to secondary infections. In addition, the clinical signs such as hemoglobinuria, fever (41°C), restlessness, and recumbency point toward systemic toxemia and organ failure.[5,6]
Postmortem examination revealed gross pathological changes consistent with a severe, multifocal bacterial infection. The findings of hepatized lungs, frothy exudates in the trachea, and congested organs, combined with hemorrhagic gastroenteritis and helminth infestation, suggest a state of overwhelming sepsis. Isolation and identification of the pathogens confirmed their involvement in the disease process. Corynebacterium spp., characterized by its unique Chinese letter-like appearance and catalase positivity, is known for its opportunistic pathogenicity in immunocompromised animals. The presence of E. coli and Klebsiella spp., both facultative pathogens, indicates possible aspiration or hematogenous spread, contributing to severe pulmonary infection and systemic dissemination.[7,8]
Antibiotic resistance profiles of the isolated bacteria further complicated the therapeutic management of the case. The resistance of all three isolates to multiple antibiotics, including commonly used agents such as ciprofloxacin, gentamicin, and amoxicillin, underscores the growing challenge of antimicrobial resistance in veterinary medicine.[9] It reiterates the importance of infection control and supportive care in managing systemic infections.[10]
CONCLUSION
This case emphasizes the critical challenge of MDR bacterial infections in equine medicine, highlighting the need for prompt diagnosis, effective treatment, and antimicrobial stewardship. It reiterates the need for ongoing surveillance and research into resistance patterns in veterinary pathogens.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
Patient’s consent not required as there are no patients in this study.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship:
Nil.
References
- Clinical Microbiology in Detection and Identification of Emerging Microbial Pathogens: Past, Present and Future. Emerg Microbes Infect. 2022;11:2579-89.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency of Detection and Prevalence Factors Associated with Common Respiratory Pathogens in Equids with Acute Onset of Fever and/or Respiratory Signs (2008-2021) Pathogens. 2022;11:759.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J Clin Lab Anal. 2022;36:e24655.
- [CrossRef] [PubMed] [Google Scholar]
- The Airway Pathobiome in Complex Respiratory Diseases: A Perspective in Domestic Animals. Front Cell Infect Microbiol. 2021;11:583600.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives. Cells. 2023;12:1052.
- [CrossRef] [PubMed] [Google Scholar]
- Causes and Pathology of Equine Pneumonia and Pleuritis in Southern Brazil. J Comp Pathol. 2020;179:65-73.
- [CrossRef] [PubMed] [Google Scholar]
- Agents Isolated from Horses with Respiratory System Infection Signs. Etlik Vet Mikrobiyol Derg. 2022;33:56-62.
- [CrossRef] [Google Scholar]
- Co-Occurrence of Multidrug Resistant Klebsiella pneumoniae Pathogenic Clones of Human Relevance in an Equine Pneumonia Case. Microbiol Spectr. 2022;10:e0215821.
- [CrossRef] [PubMed] [Google Scholar]
- Improving Clinical Outcomes via Responsible Antimicrobial Use in Horses. Equine Vet Educ. 2021;34:482-92.
- [CrossRef] [Google Scholar]






